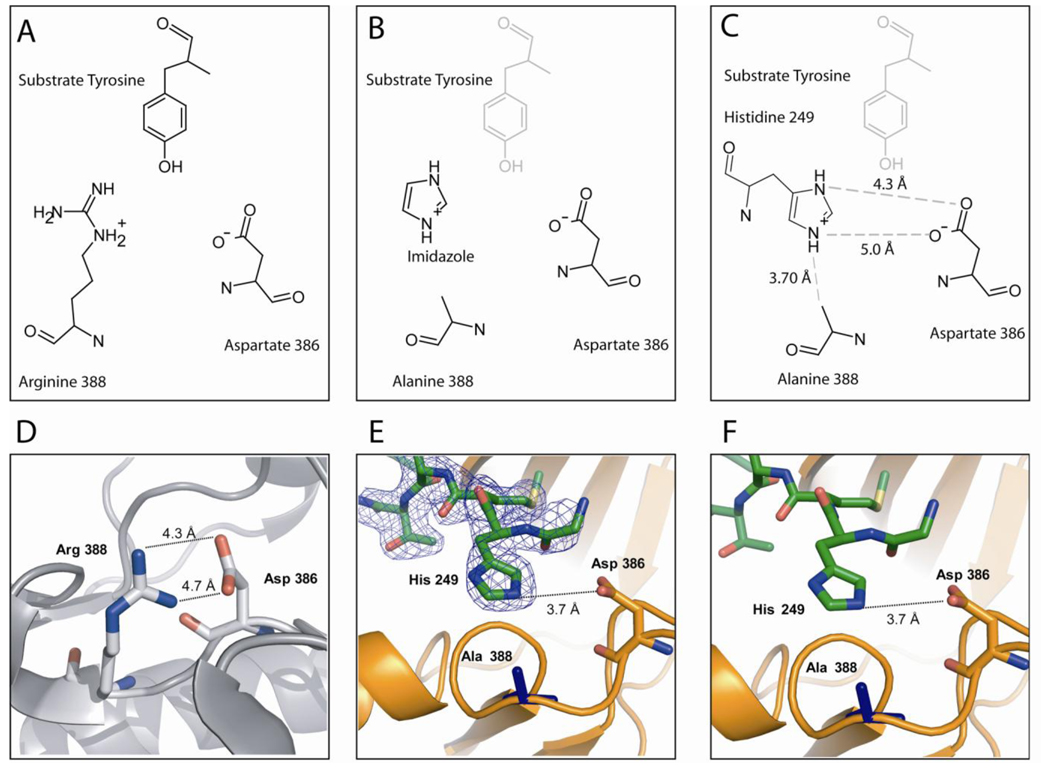
Figure 3. Occupation of Histidine in R388A SrcKD Active Site.
(A) Orientation of the catalytic residues and substrate tyrosine in the structure of insulin receptor kinase (PDB entry 1IR3; Src numbering) (19). (B) Model for chemical rescue of R388A Src by imidazole as proposed by Qiao et al.(12). The protonated imidazolium fulfills the hydrogen bonding network between the catalytic residues and the susbtrate tyrosine. (C) Schematic drawing of the coordination of His249 by the active site residues. (D) Distances between the active site residues of wild-type Src in the absence of substrate peptide (PDB entry 1Y57). (E, F) Structure of R388A SrcKD with electron density of the 2F0-Fc contoured to 1σ. The His249 residue of a symmetry related molecule (green) inserts into the active site of a kinase (orange). The distances for this structure are virtually identically to the distances in the wild-type Src structure, which is also free of susbstrate peptide.