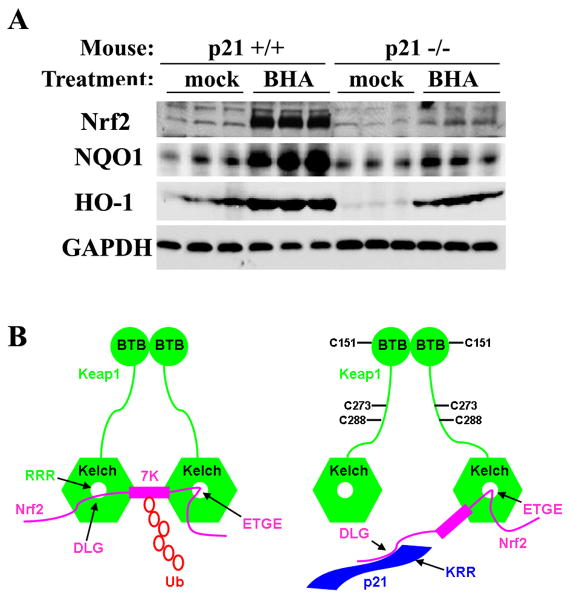
Figure 7. p21-Deficient Mice had Reduced Basal and Induced Levels of Nrf2 and Nrf2 Target Genes.
(A) p21-deficient mice had reduced basal and induced levels of Nrf2 and Nrf2 target genes. Wild-type or p21-deficient mice (n=3) were treated with 350 mg/kg BHA for 12 hrs through intraperitoneal injection. Liver tissues were subjected to immunoblot analysis with anti-Nrf2, anti-NQO1, anti-HO-1, and anti-GAPDH antibodies. (B) A model by which p21 upregulates the Nrf2-dependent antioxidant response at both basal and induced conditions. Keap1, an E3 ubiquitin ligase containing the BTB and Kelch domains, constantly targets Nrf2 for ubiquitination and subsequent degradation under basal conditions. The binding of Keap1 to Nrf2 is through a hinge and latch mechanism, in which each Kelch domain from a Keap1 homodimer binds Nrf2 through two binding sites: a weak-binding DLG motif and a strong-binding ETGE motif. Binding of both sites are essential to present the seven ubiquitin-accepting lysine residues of Nrf2 in the correct orientation to accept ubiquitin, which targets Nrf2 for degradation. In response to oxidative stress, Keap1 is able to detect an imbalance in intracellular redox homeostasis through modification of its cysteine residues. The three important cysteine residues, C151, C273, and C288 are labeled. These modifications alter conformation of Keap1 and loosen the latch (DLG-Kelch), which puts p21 in a better position to compete with Keap1 for binding to the DLG motif. Once the latch is open, the Keap1-dependent ubiquitination of Nrf2 is compromised even though Keap1 still associates with Nrf2 through the hinge (ETGE). Thus, Nrf2 is stabilized and Nrf2-dependent cytoprotective genes are expressed under oxidative stress.