Abstract
Purpose
To describe the clinical, morphologic, and immunohistochemical features of a case of paranasal natural killer/T-cell lymphoma (NKTL) with ocular involvement.
Case report
In March 2005 the patient presented with a maxillary sinusitis and upper nasal obstruction. In July she underwent a nasal computed tomography (CT) scan and multiple biopsies of the granulomatous tissue in the nasal fossae. The diagnosis was NK/T non-Hodgkin's lymphoma nasal type, stage IV A. Afterwards she presented anterior uveitis. In September after the diagnosis of lymphoma the patient underwent a bone marrow biopsy and thoracic and abdominal CT scan. An ophthalmic examination including visual acuity assessment and fundoscopic examination was made. In October she started chemotherapy cycles. Maxillary CT scan and ophthalmic examinations were performed during the cycles. In January 2006 after severe recurrences of panuveitis a diagnostic vitrectomy was performed.
Results
A diagnosis of T-lymphoma cells in the vitreous was made; the tumor was most likely originating from her paranasal NKTL. The condition of the patient deteriorated rapidly and she expired on February 2006.
Conclusions
Nasal and paranasal sinus lymphomas are rare, but aggressive diseases with a tendency to invade tissues and spread to CNS, including the eye. Ocular manifestations prior to systemic ones may be useful to monitor the response to therapy.
Keywords: Masquerade syndrome, Nasal type lymphoma, Natural killer T-cell lymphoma, Serous retinal detachment
Introduction
Under the World Health Organization (WHO) Classification, extranodal nasal natural killer/T-cell lymphoma (NKTL) is a form of lymphoma derived from natural killer (NK) cells and/or cytotoxic T-lymphocytes that typically involves the nasal cavity and paranasal sinuses [1]. Nasal NKTL is a definitive diagnostic entity included in the WHO lymphoma classification [2]. It is a disease common among Oriental, Native American, and Hispanic patients, and is associated with the Epstein-Barr virus (EBV) [3]. More than 70% of cases localize in the nasopharyngeal region, although extranasal and disseminated disease can occur [4]. Owing to anatomic proximity, nasal NKTL can lead to ocular complications. However, ocular complications in pathologically confirmed cases are rarely reported [5, 6].
Intraocular lymphomas of non-B-cell type are rare and represent approximately 1–3% of all lymphoproliferative lesions in this site [7–10]. Furthermore, lymphomas expressing NK-cell markers are known to have a highly aggressive behavior and a poorer prognosis than B-cell lymphoma or T-cell lymphoma [10–12]. We present the clinical, morphologic, immunohistochemical, and molecular features of a case of paranasal NKTL with ocular involvement.
Case report
A 54-year-old Caucasian female presented in March 2005 with a maxillary sinusitis and upper nasal obstruction. She was treated with antibiotics. Two months later she developed hypo-anesthesia of the palate durum and upper lip. A computed tomography (CT) scan in July 2005 showed abundant tissue in the nasal fossa and in the left maxillary sinus. She underwent multiple biopsies of the granulomatous tissues in the nasal fossae and was finally diagnosed with NK/T non-Hodgkin's lymphoma nasal type, stage IV A, EBV positive in September 2005. At the same time she showed left hand paraesthesia. Immediately after the nasal biopsies she presented anterior uveitis in the right eye (RE) and was treated with topical dexamethasone.
From the end of September and early October, her sinus CT demonstrated thickening maxillary mucosa in the right and was full of isodense soft tissue in the left maxillary sinuses. Furthermore, there was a thickening of fossae nasalis mucosa and demineralized turbinate. Reactive lymph nodules were also detected by mammography. The thoracic and abdominal CT scans as well as bone marrow biopsy were all negative. Ophthalmic examination disclosed visual acuities of 0.2 in RE and 1.0 in left eye (LE). Fundoscopic examination revealed vitritis and retinochoroideal infiltration (non-rhegmatogenous detachment) in RE and normal in LE (Figs. 1–2).
Fig. 1.
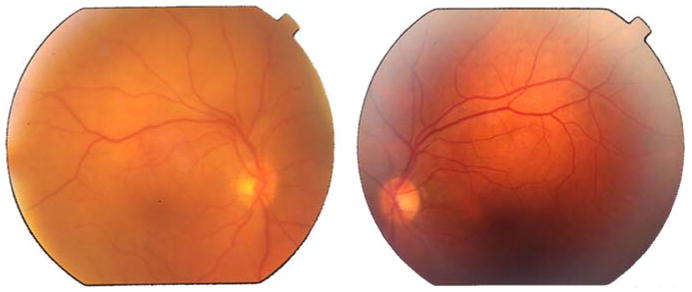
The fundus examination of the posterior pole shows mild vitritis only in the right eye
Fig. 2.
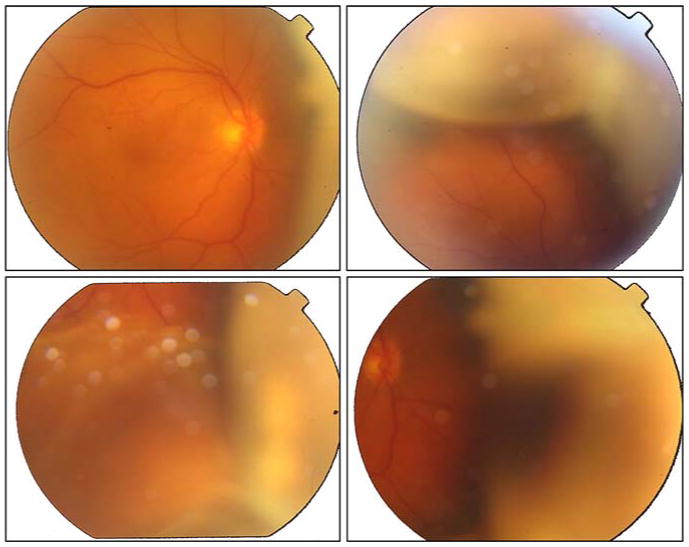
Right eye fundus (in mydriasis). The fundus examination confirmed retinal and choroideal serous detachment in the right eye
On 7 October 2005, the patient received chemotherapy (CHOP = cyclophosphamide, vincristine, adriamycin, prednisolone) in 14-day cycles plus central nervous system (CNS) prophylaxis with 10 mg/week (for 5 weeks) methotrexate. On 31 October after two chemotherapy cycles her maxillary CT showed significant improvement in spite of remaining lower opacities in the left maxillary sinus and nasal fossa. The right maxillary sinus opacity had totally disappeared. The patient had also a favorable ophthalmic response with improving vision to 0.6 in RE and significant decrease of vitritis and retinochoroideal infiltration. The LE was normal. On 21 November, after the fourth cycle of CHOP, she had a negative total body positron emission tomography (PET). The therapeutic plan was continuous chemotherapy with eight CHOP 14-day cycles after radiotherapy.
On 19 December after the sixth cycle of CHOP, the patient developed a recurrent non-granulomatous panuveitis in both eyes (RE > LE) with vitritis 2+ in RE, and she was treated with systemic corticosteroid without any response. After the seventh CHOP cycle (2 January 2006), the patient's clinical symptoms worsened with fever accompanied by increased swelling of the right periorbital and facial area. She also presented with intense vitreitis 3+ RE. On 17 January the patient underwent systemic workup including normal thorax CT scan and negative blood culture. Spinal cord fluid was positive for EBV.
A diagnostic vitrectomy was performed on 25 January. The specimen was evaluated at the National Eye Institute, Bethesda, MD, and disclosed a few atypical large lymphoid cells (Fig. 3) admixed with many erythrocytes and some inflammatory cells (macrophages, small lymphocytes and occasional neutrophils). The atypical lymphoid cells stained positive for CD3 and CD56, negative for CD20, and were detected to have TCR-gamma gene rearrangements using the primer pairs of T-γ 177/178 (Fig. 4). The sample contained high levels of IL-10 (743 pg/ml), IL-6 (311 pg/ml), and TGF-β (1,185 ng/ml). Vitreous was found positive for EBV DNA when performed in the hospital of Reggio Emilia (PCR method). A diagnosis of T-lymphoma cells in the vitreous was made; the tumor was most likely originating from her paranasal NKTL. The high vitreous levels of TGF-β and IL-10 as well as immunoreactivity against CD56 and positive TCR-γ rearrangements supported the diagnosis. Unfortunately, the patient's condition deteriorated rapidly with the development of liver and kidney failure. She expired on 4 February 2006.
Fig. 3.
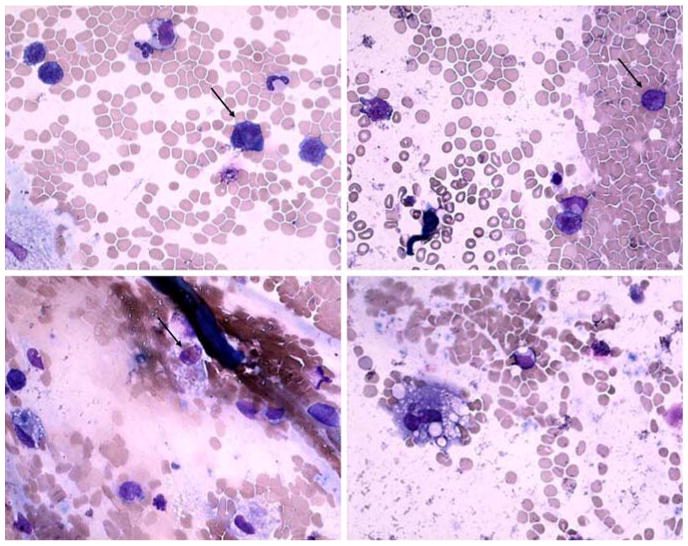
Vitreous sample illustrates few large atypical lymphoid cells with large polymorphic nuclei and scanty basophilic cytoplasm
Fig. 4.
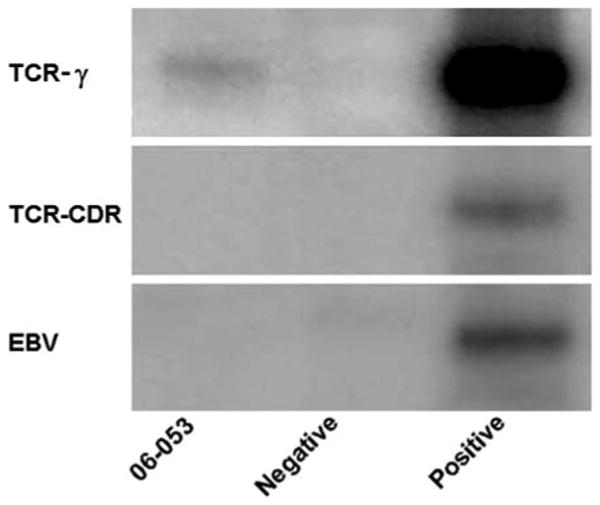
PCR amplification shows TCR-gamma gene rearrangements in the lymphoma cells from the vitreous (NIH; Bethesda, MD)
Discussion
According to the WHO classification, extranodal NKTL, nasal and paranasal type, is characterized by a lymphoid infiltrate of varying morphology composed primarily of NK cells (CD56+) [1, 2]. The cellular infiltrate generally demonstrates evidence of EBV infection [3]. The nasal type is the most common site of this malignancy, explaining the use of the term nasal type in the WHO nomenclature. Nasal NKTL is more frequent in Asia and Central America, and is relatively rare in the US and Europe [13]. Lymphomas arising primarily in the nasal cavity are mostly of T-cell origin and those in the paranasal sinus are more of B-cell origin [14]. Primary nasal lymphomas are mostly of NK cell origin. Interestingly, our patient presented with maxillary sinus lymphoma of NKTL classification.
Non-Hodgkin's lymphoma of the paranasal sinus is an uncommon presentation of extranodal lymphoma. Its natural history, treatment and prognosis have been infrequently characterized; however, a tendency to involve the CNS has been reported [15]. The incidence of CNS involvement ranges from less than 1 to 30% [16–19]. In a recent study of 44 patients with paranasal sinus lymphoma from British Columbia, Canada, 84% was diagnosed with diffuse large B-cell lymphoma and 8% with NKTL [15]. Introduction of intrathecal chemoprophylaxis was associated with improvement of overall survival from 20 to 51% and of disease-specific survival from 40 to 65%. It is not surprising that nasal and paranasal sinus lymphomas are aggressive diseases with a tendency to invade tissues and metastasize to CNS. Since primary CNS lymphoma is closely related to primary intraocular lymphoma and the close location between nasal cavity, paranasal sinus, and the eye, it is predictable to observe ocular metastasis.
Intraocular T-cell or NKT cell lymphomas are extremely rare and mostly represent a secondary manifestation of either a cutaneous or systemic lymphoma [20]. In a large series of 24 cases with primary nasal and paranasal sinus NKT lymphoma, six patients suffered from vision-threatening complications of uveitis/vitritis and orbital involvement including one with rhegmatogenous retinal detachment and one with macular hole [4]. Therefore, ocular complications were relatively high (25%) and required ophthalmic treatment. Generally, the prognosis was grave, but remission could still be achieved with aggressive combined chemotherapy and radiotherapy. Our patient also presented with ocular prior to systemic manifestations. Baseline and regular ophthalmic assessments are warranted for all nasal and paranasal sinus lymphomas, in particular, the NKT lymphoma.
Contributor Information
Luca Cimino, Department of Ophthalmology, Arcispedale S.M. Nuova, Viale Risorgimento 80, 42100 Reggio Emilia, Italy, e-mail: l.cimino@libero.it.
Chi-Chao Chan, Laboratory of Immunology, National Eye Institute, Bethesda, MD, USA.
DeFen Shen, Laboratory of Immunology, National Eye Institute, Bethesda, MD, USA.
Luciano Masini, Department of Hematology, Arcispedale S.M. Nuova, Reggio Emilia, Italy.
Fiorella Ilariucci, Department of Hematology, Arcispedale S.M. Nuova, Reggio Emilia, Italy.
Maurizio Masetti, Department of Otolaryngology, Arcispedale S.M. Nuova, Reggio Emilia, Italy.
Silvia Asioli, Department of Pathology, Arcispedale S.M. Nuova, Reggio Emilia, Italy.
Antonio Sartori, Department of Ophthalmology, Arcispedale S.M. Nuova, Viale Risorgimento 80, 42100 Reggio Emilia, Italy.
Luca Cappuccini, Department of Ophthalmology, Arcispedale S.M. Nuova, Viale Risorgimento 80, 42100 Reggio Emilia, Italy.
References
- 1.Chan JKC, Jaffe ES, Ralfkiaer E. Extranodal NK/T-cell lymphoma, nasal type. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organizzation classification of tumours: tumours of haematopoietic and lymphoid tissues. IARC; Lyon: 2001. pp. 204–207. [Google Scholar]
- 2.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 3.Kwong YL, Chan AC, Liang RH. Natural Killer cell lymphoma/leukaemia: pathology and treatment. Hematol Oncol. 1997;15:71–79. doi: 10.1002/(sici)1099-1069(199705)15:2<71::aid-hon601>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Hon C, Kwok AK, Shek TW, Chim JC, Au WY. Vision-threatening complications of nasal T/NK lymphoma. Am J Ophthalmol. 2002;134:406–410. doi: 10.1016/s0002-9394(02)01520-9. [DOI] [PubMed] [Google Scholar]
- 5.Coupland SE, Krause L, Delecluse HJ, et al. Lymphoproliferative lesions of the ocular adnexa. Analysis of 12 cases. Ophthalmology. 1998;105:1430–1441. doi: 10.1016/S0161-6420(98)98024-1. [DOI] [PubMed] [Google Scholar]
- 6.Meyer JH, Scharf B, Gerling J. Midline granuloma presenting as orbital cellulites. Graefes Arch Clin Exp Ophthalmol. 1996;234:137–139. doi: 10.1007/BF00695254. [DOI] [PubMed] [Google Scholar]
- 7.Jakobiec FA, Lefkowitch J, Knowles DM. II. B- and T-lymphocytes in ocular disease. Ophthalmology. 1984;91:635–654. doi: 10.1016/s0161-6420(84)34256-7. [DOI] [PubMed] [Google Scholar]
- 8.Peterson K, Gordon KB, Heinemann MH, De Angelis LM. The clinical spectrum of ocular lymphoma. Cancer. 1993;72:843–849. doi: 10.1002/1097-0142(19930801)72:3<843::aid-cncr2820720333>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 10.Chan CC, Wallace DJ. Intraocular lymphoma: update on diagnosis and management. Cancer Control. 2004;11:285–295. doi: 10.1177/107327480401100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clearly KR, Batsakis JG. Sinonasal lymphomas. Ann Otol Rhinol Laryngol. 1994;103:911–914. doi: 10.1177/000348949410301116. [DOI] [PubMed] [Google Scholar]
- 12.Jaffe ES, Chan JKC, Su U, et al. Report of the workshop on nasal and related extranodal angiocentric T/Natural Killer cell lymphomas: definitions, differential diagnosis and epidemiology. Am J Surg Pathol. 1996;20:103–111. doi: 10.1097/00000478-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Macon WR, William ME, Greer JP, et al. Natural killer-like T cell lymphoma; aggressive lymphomas of T-large granular lymphocytes. Blood. 1996;87:1474–1483. [PubMed] [Google Scholar]
- 14.Kitamura A, Yamashita Y, Hasegawa Y, Kojima H, Nagasawa T, Mori N. Primary lymphoma arising in the nasal cavity among Japanese. Histopathology. 2005;47:523–532. doi: 10.1111/j.1365-2559.2005.02265.x. [DOI] [PubMed] [Google Scholar]
- 15.Laskin JJ, Savage KJ, Voss N, Gascoyne RD, Connors JM. Primary paranasal sinus lymphoma: natural history and improved outcome with central nervous system chemoprophylaxis. Leuk Lymphoma. 2005;46:1721–1727. doi: 10.1080/17402520500182345. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs C, Hoppe RT. Non-Hodgkin's lymphomas of head and neck extranodal sites. Int J Radiat Oncol Biol Phys. 1985;11(2):357–364. doi: 10.1016/0360-3016(85)90158-0. [DOI] [PubMed] [Google Scholar]
- 17.Frierson HF, Jr, Mills SE, Innes DJ., Jr Non-Hodgkin's lymphomas of the sinonasal region: histologic subtypes and their clinicopathologic features. Am J Clin Pathol. 1984;81(6):721–727. doi: 10.1093/ajcp/81.6.721. [DOI] [PubMed] [Google Scholar]
- 18.Tran LM, Mark R, Fu YS, Calcaterra T, Juillard G. Primary non-Hodgkin's lymphomas of the paranasal sinuses and nasal cavity. A report of 18 cases with stage IE disease. Am J Clin Oncol. 1992;15:222–225. doi: 10.1097/00000421-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Logsdon MD, Ha CS, Kavadi VS, Cabanillas F, Hess MA, Cox JD. Lymphoma of the nasal cavity and paranasal sinuses: improved outcome and altered prognostic factors with combined modality therapy. Cancer. 1997;80:477–488. doi: 10.1002/(sici)1097-0142(19970801)80:3<477::aid-cncr16>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Coupland SE, Joussen A, Anastassiou G, Stein H. Diagnosis of a primary uveal extranodal marginal zone B-cell lymphoma by chorioretinal biopsy: case report. Graefes Arch Clin Exp Ophthalmol. 2005;243:189–197. doi: 10.1007/s00417-004-1050-4. [DOI] [PubMed] [Google Scholar]


