Figure 3.
In Vivo and in Vitro Binding of the bZIP53 Protein to the Albumin 2S2 Promoter and Interaction with bZIP10 and bZIP25.
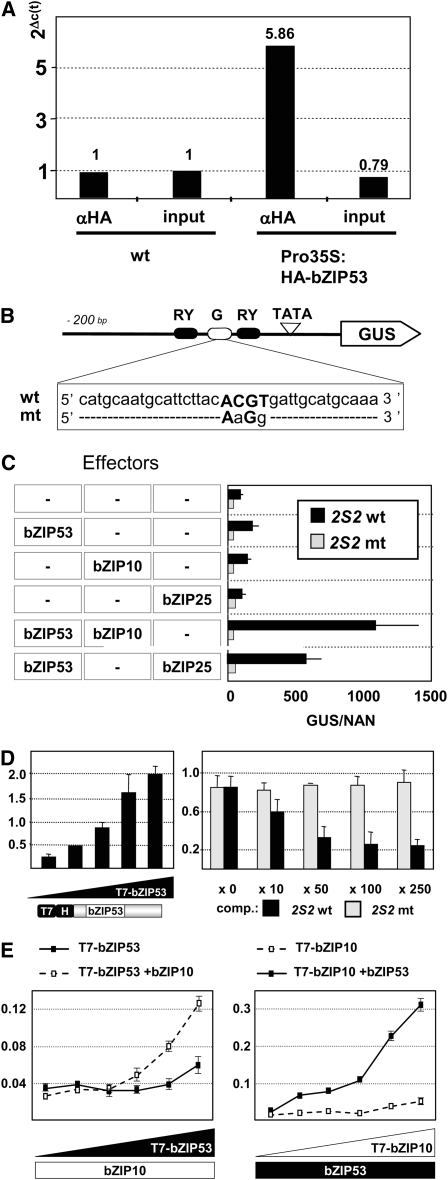
(A) Chromatin extracts from wild-type plants and plants overexpressing a HA-tagged bZIP53 protein (Pro35S:HA-bZIP53) were subjected to qRT-PCR analysis with 2S2 promoter-specific primers before (input) and after immunoprecipitation with an anti-HA antibody (α-HA). Ct values for Pro35S:HA-bZIP53 samples were subtracted from the Ct values of the equivalent wild type, and the differentials are shown on top of the right bars in the graph. A value of 1 was assigned to the Col-0 samples. For normalization, an actin (ACT7) gene was used (see Methods).
(B) Schematic view of the 2S2 promoter fused to GUS used as reporter in transient expression analysis. Depicted are RY (black) and G-box (white) elements. The sequence of the wild type and mutated G-box (mt) is shown.
(C) Arabidopsis leaves were transformed with the reporter constructs containing sequences described in (B) and effector constructs containing specific bZIP genes under control of the Pro35S in cobombardment experiments as described by Lara et al. (2003). Three microliters of control plasmid Pro35S:NAN was included in all the experiments to normalize GUS expression values for differences in bombardment efficiencies (Kirby and Kavanagh, 2002). The x axis values are expressed as GUS activity relative to NAN. Given are mean values and standard deviation of three independent experiments.
(D) In vitro binding of bZIP53 to the G-box sequence from the 2S2 promoter. A biotinylated oligonucleotide containing the G-box sequence was bound to streptavidin-coated wells and incubated with increasing amounts of a T7-tagged bZIP53 protein (1 to 1:90). Nonbound proteins were removed from the reaction wells, and the amount of T7-bZIP53 protein was quantified by immunodetection with an anti-T7 antibody (α-T7; left panel). The binding specificity of bZIP53 to the 2S2 G-box was analyzed in competition experiments where increasing amounts of unlabeled oligonucleotides (as indicated) containing a wild-type (2S2 wt; black bars) or a mutated 2S2 mt version (gray bars) were incubated with a fixed amount of the T7-bZIP53 protein and the biotinylated oligonucleotide containing the wild-type G-box sequence (right panel).
(E) Effect of heterodimerization on bZIP53 and bZIP10 binding affinity. A biotinylated oligonucleotide containing the G-box sequence was bound to streptavidin-coated wells and incubated with increasing amounts (1 to 1:30) of a T7-bZIP53 protein in the absence (filled squares) or presence of a fixed amount of bZIP10 protein (open squares). Nonbound proteins were removed from the reaction wells, and the amount of T7-bZIP53 protein was quantified by immunodetection with an α-T7 antibody (left panel). The reciprocal experiment was performed with a T7-bZIP10 protein and a nontagged bZIP53 protein (right panel). Given are mean values and standard deviation of three repetitions.