Figure 4. Generation of genetically unmodified germline competent NOD ES cells.
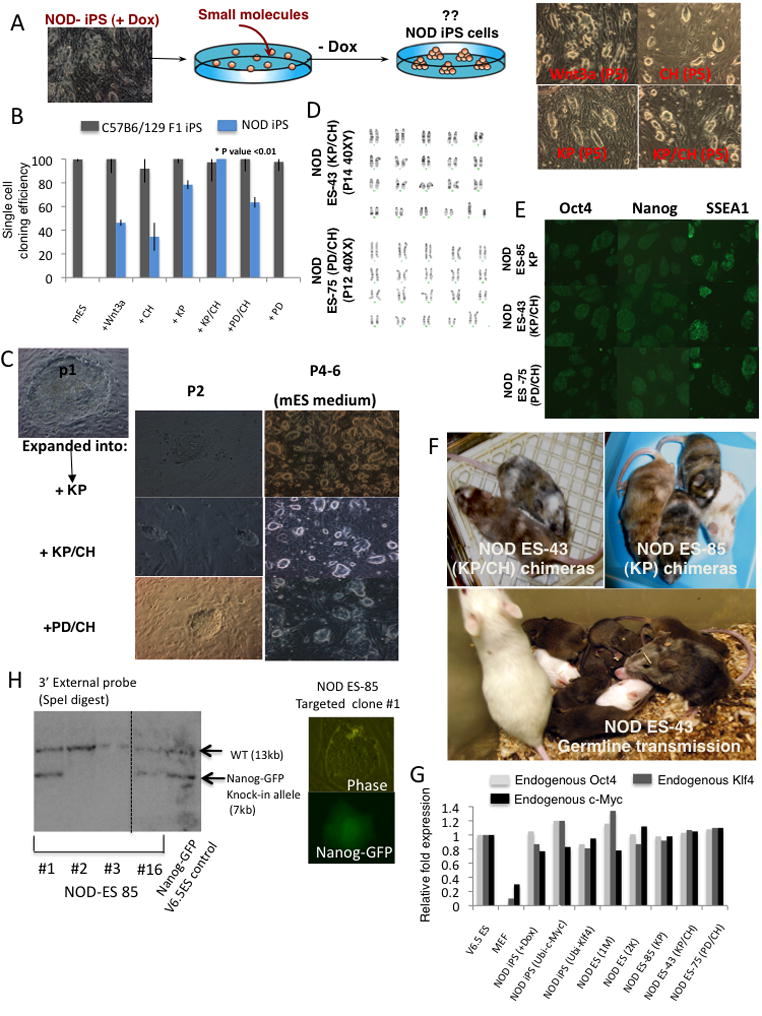
(A) Experimental outline for derivation of iPS lines that grow in the presence of small molecules and in the absence of Dox. Representative images of NOD iPS lines growing in mESM supplemented with the indicated small molecules or growth factors. (B) Single-cell cloning efficiency of NOD iPS cells in different growth conditions. WT or NOD-iPS cells grown in mESM and KP were sorted in 96 well plates and cultured in mESM supplemented with small molecules. The number of wells containing Nanog+ colonies was counted after 6 days. Efficiencies were normalized to that of C57B6/129F1-iPS cells (“permissive” controls) plated in mESM and defined as 100%. SD for average efficiencies from 2 experiments are shown. Student ttest P value compares KP/CH to PD/CH mESM conditions for NOD iPS cells. (C) Expansion of day E3.5 ICM derived NOD ES cells in the presence of KP, KP/CH or PD/CH and derivation of stable ES lines. Images of initial colonies observed at passage 2 after embryo dissociation are shown together with representative images at later passages. (D) Karyotype of NOD ES lines. (E) Immunostaining of NOD ES lines for pluripotency markers. Inhibitors used during cell line derivation and propagation are indicated. (F) Chimerism in adult mice generated from the indicated cell lines is evident by the presence of agouti (brown) and albino coat color originating from the NOD background. Lower panel demonstrates germline transmission obtained from a male NOD ES #43 derived chimera that was mated to an NOD female. (G) Real-Time PCR analysis for endogenous expressed Oct4, c-Myc and Klf4 genes in NOD iPS and ES cells grown in the indicated conditions. Relative expression levels are normalized to levels detected in control 129 ES cells. (H) Southern analysis indicating correct targeting of the endogenous Nanog locus in NOD ES cells with Nanog-GFP knock-in targeting construct.
