Abstract
Changes in hepatitis C virus (HCV) prevalence from 1992 to 2006 were examined by using 24,311 records from unlinked anonymous surveillance of injecting drug users in England and Wales. Bayesian logistic regression was used to estimate annual prevalence, accounting for changing recruitment patterns (age, gender, injecting duration, geographic region, interactions) and the sensitivity and specificity of different oral fluid testing devices. After controlling for these differences, the authors found that the adjusted HCV prevalence decreased from 70% (95% credible interval: 62, 78) in 1992 to 47% (95% credible interval: 43, 51) in 1998 before rising again to 53% (95% credible interval: 48, 58) in 2006. Women injecting drug users had a higher HCV risk than did men (odds ratio = 1.50, 95% credible interval: 1.31, 1.73). Two regions (London and North West) had a markedly higher HCV prevalence than did the rest of England and Wales. Among individuals who had injected for less than 1 year, the adjusted HCV prevalence in 2006 was higher than that in 1992 (28% vs. 19%, respectively). HCV infection can be prevented. The public health challenge in England and Wales is to increase action in order to regain a downward trend in HCV risk and the benefit that has been lost since 1998.
Keywords: England; hepatitis C; prevalence; sentinel surveillance; substance abuse, intravenous; Wales
Hepatitis C virus (HCV) infection is preventable and treatable. In most developed and transitional countries, HCV transmission has been concentrated among injecting drug users (IDUs) (1–4), who typically experience high rates of infection. For example, in England and Wales, an estimated 80% of all HCV cases are due to injecting drug use (5, 6). Preventing infection in this population is thus a key component of public health responses to reduce HCV-related harm and morbidity. The main primary interventions include the provision of sterile injecting equipment and reducing and stopping injection through opiate substitution therapy (7, 8). Interpreting HCV surveillance data in order to assess the impact of prevention is complicated by difficulties associated with recruiting representative samples of IDUs. Although better methods are being developed (9), most survey data will suffer from recruitment or selection bias, which needs to be properly accounted for when comparing trends in HCV prevalence over time.
Although many studies have reported a high HCV prevalence among IDUs, few have reported trends over time, and these have revealed contrasting pictures. In Australia, the prevalence of human immunodeficiency virus (HIV) is low, the availability of interventions is good, but HCV prevalence remains persistently above 50% and has increased among recent injectors (10). In Glasgow, Scotland, HCV prevalence was estimated to have fallen during the early 1990s but remains above 50% (11). In the United States, evidence for a decrease in HCV prevalence has been found in a number of cities (Baltimore, Maryland; Los Angeles, California; and New York, New York) (12, 13).
In this paper, we use surveillance data collected from approximately 50 sampling sites per year from 1992 to 2006 and comprising 24,311 participations. We examine trends in HCV prevalence and investigate whether, after adjustment for changing recruitment patterns (age at test, gender, injecting duration, geographic region, interactions) and test accuracy, HCV prevalence has declined.
MATERIALS AND METHODS
The survey
A national unlinked anonymous surveillance of IDUs was started in 1990 to monitor trends in HIV infection by using specialist services for IDUs as a pragmatic sampling frame. Detailed methods have been published elsewhere (14, 15). Briefly, voluntary oral-fluid samples and an accompanying questionnaire are collected annually from consenting IDUs attending representative services across England and Wales providing needle exchange, opiate substitution therapy, and treatment for drug use. Since 1998, the samples have been routinely tested for anti-HCV antibodies, and a recent extension has allowed retrospective anti-HCV testing of stored samples from the 1992, 1994, and 1996 surveys. Those aged 15–59 years who had injected in the previous 4 weeks were included in the analyses in this paper.
Estimating trends in anti-HCV prevalence presents a number of difficulties. First, the participating services and their geographic distribution have changed over time, reflecting changing patterns of drug use and service provision. For example, in the earlier years, there were many services in the North West region of England, an area with historically high rates of HCV infection among IDUs but also one of the areas most affected by injecting drug use in the early 1990s (16, 17). Hence, regional variations in data collection occur over time and must be adjusted for in the analysis. Second, the device used to collect oral fluids has also changed over time, and this affects the sensitivity and specificity of the assay in detecting anti-HCV antibodies. Prior to 1998, the Salivette (Sarstedt, Ltd., Leicester, United Kingdom) collection device was used in the survey, while from 1998 onward, the OraSure (OraSure Technologies, Inc., Bethlehem, Pennsylvania) device has been used. In a population whose anti-HCV prevalence is 30%, the Salivette device has been estimated to have a sensitivity of only 74.1% (95% confidence interval (CI): 68.2, 79.4) with 99.0% (95% CI: 97.4, 99.7) specificity, while the OraSure device has an estimated sensitivity of 91.7% (95% CI: 87.5, 94.8) and specificity of 99.2% (95% CI: 97.8, 99.8) (18). Therefore, the sensitivity of detecting anti-HCV is higher in the 1998–2006 surveys compared with those conducted before 1998.
This paper uses logistic regression to estimate the shape of the prevalence trend, while adjusting for variables that may explain the changing recruitment in unlinked anonymous data. The analysis accounts for differential misclassification of the infection status; that is, the sensitivity and specificity are different for samples collected before and since 1998. Failure to acknowledge sensitivities and specificities in the analysis would result in an underestimate of anti-HCV prevalence and a severe underestimation in the years prior to 1998.
Regression model
A classical logistic regression model is first implemented, with the observed anti-HCV test result as the binary response variable. This is regressed against the year in which the test is taken in order to estimate a period effect. To account for changing recruitment effects, the following demographic variables are included in the analysis: age, gender, injecting duration, and geographic region of recruitment (London, Midlands and Wales, North East and Yorkshire, North West, Southern and Eastern). We do not attempt to adjust for other variables that may explain the period effect, for example, injecting risk behaviors. Adjustment for these variables will dilute the HCV risk trend that we are attempting to infer.
Nonlinear relations are investigated for all continuous variables (year of test, age, injecting duration) through the use of polynomials. For each variable, the degree of the polynomial is chosen by selecting the model with the lowest Akaike Information Criterion (AIC). Polynomial functions up to degree 4 (quartic) were assessed for each continuous variable. To further improve the fit of the model, all 10 possible 2-way interactions of the 5 variables are considered. Variable selection is performed, and the model with the lowest AIC is chosen. A global goodness-of-fit test for the final binary logistic model is performed by using the method proposed by le Cessie and van Houwelingen (19). To judge the effect of missing data on model estimates, we conducted multiple imputation using chained equations (20) in a sensitivity analysis. The prevalence trend estimated from the final model is plotted over the study period by setting all other covariates to their mean values in the data set.
Prevalence in recent initiates (those with injecting duration of <1 year) is strongly associated with HCV incidence, and hence we also considered an additional model where injecting duration is expressed as a binary variable (recent vs. nonrecent initiates).
Accounting for imperfect sensitivity and specificity of the test device
As discussed above, the anti-HCV test sensitivity and specificity are not perfect and differ over time as the result of a change in the testing device. From the law of total probability, a positive test result occurs with probability,
where π is the true prevalence of disease, and “Sens” and “Spec” are the sensitivity and specificity of the test, respectively. If the sensitivity and specificity are known exactly, then one can estimate the true probability of disease from the observed test results. Magder and Hughes (21) suggest an expectation-maximization algorithm to do this in the context of logistic regression.
In this setting, the sensitivity and specificity of the assay using the 2 collection devices are not known exactly, and we wish to incorporate this uncertainty in the model. Their estimated values together with 95% confidence intervals are given above and as reported by Judd et al. (18). To incorporate this additional uncertainty, a Bayesian analysis is implemented with the same choice of variables as from the best-fitting classical logistic model. The sensitivity and specificity of each device are given informative prior probability distributions to reflect the uncertainty surrounding their values. To achieve this, the log-odds of both “Sens” and “Spec” are assumed to follow normal distributions with means equal to the log-odds of the estimated values, and variances were calculated from the corresponding confidence intervals. Regression parameters in the logistic model are given noninformative prior distributions, and inferences are made from the posterior distributions of these parameters. A similar approach is described in detail by McInturff et al. (22). The Bayesian analysis provides a posterior distribution for each parameter of the logistic model and, hence, a posterior distribution for the predicted HCV prevalence from each combination of covariate values and for differences in HCV prevalence between subgroups. This, in turn, allows calculation of posterior probabilities of any quantity of interest, for example, the difference between HCV prevalence in 2006 compared with that in 1992.
Implementation
All classical analyses were carried out by using the “glm” function in R. Bayesian analyses were performed by using Markov chain-Monte Carlo techniques implemented in WinBUGS (23), and for which the model code is available from the authors upon request. Inferences were based on 32,000 iterations from 2 chains running in parallel, and convergence was assessed through the use of the Brooks-Gelman diagnostic (24).
RESULTS
In total, 24,311 participations were available from England and Wales over the years 1992, 1994, 1996, and 1998–2006. Appendix Table 1 in the Appendix shows the observed number and proportion testing positive for anti-HCV by year, sex, region, injecting duration, and age.
The final classical logistic regression model (with the lowest AIC) included all main effects and six 2-way interactions (year of test × region, year of test × injecting duration, region × injecting duration, region × gender, region × age, gender × injecting duration). The main effects of the continuous covariates, year of test and injecting duration, were found to be best modeled by use of cubic polynomials, while age was found to be best modeled by use of a quadratic. The final model showed a good fit to the data (global test for lack of fit, P = 0.862). Parameter estimates from this model are shown in the Appendix (Appendix Table 2).
There were 1,087 (4.5%) individuals missing information on injecting duration, 504 (2.1%) missing HCV test status, 465 (1.9%) missing age, 140 (0.6%) missing gender, and 2 (0.008%) missing region of recruitment. All individuals missing anti-HCV test status were from participations in 1992, 1994, or 1996 (stored sample either could not be located or was insufficient for testing). A multiple imputation analysis was undertaken, and results were almost identical to those from the complete case analysis (results not shown but available on request); therefore, the findings from the unimputed analyses are reported here.
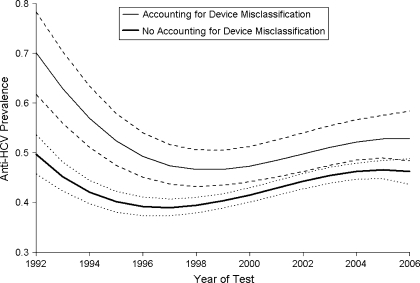
Figure 1 shows the change in estimated prevalence from the Bayesian logistic model before and after accounting for the sensitivity and specificity of the oral-fluid test. Accounting for anti-HCV test misclassification has the effect of increasing the estimated prevalence, especially before 1998, when the sensitivity was lower. The uncertainty surrounding the estimated prevalence also increases to account for the uncertainty in the level of test sensitivity and specificity. In Figure 1, the estimated prevalence is shown, with all the other variables set to their mean levels, and can therefore be interpreted as the average adjusted prevalence in the study population. Results suggest that the adjusted HCV prevalence among IDUs decreased from 70% (95% credible interval: 62, 78) in 1992 to 47% (95% credible interval: 43, 51) in 1998, before slowly rising again to a level of 53% (95% credible interval: 48, 58) in 2006. Using this predicted trend, we estimate a 99.9% chance that the HCV prevalence in 2006 is greater than that in 1998.
Figure 1.
Estimated hepatitis C virus (HCV) antibody prevalence trend for injecting drug users before and after accounting for test device sensitivities and specificities, England and Wales, 1992–2006. All other covariates are set to their mean values. Dashed lines show 95% credible intervals for the model accounting for device misclassification. Dotted lines show 95% credible intervals for the model not accounting for device misclassification.
Table 1 shows the odds ratio of HCV infection for region, gender, injecting duration, and age, when all other covariates are at their mean level. After adjustment, there is evidence for higher HCV prevalence among female IDUs than among males (odds ratio = 1.50, 95% credible interval: 1.31, 1.73). HCV prevalence also increases with injecting duration, with the odds ratio for those injecting for 5 years compared with <1 year estimated as 2.84 (95% credible interval: 2.47, 3.38).
Table 1.
Multivariate Bayesian Model of Hepatitis C Virus Infection Risk for Injecting Drug Users, England and Wales, 1992–2006
| Variable | Odds Ratioa | 95% Credible Interval |
| Region | ||
| London | 1.00 | |
| Midlands and Wales | 0.47 | 0.39, 0.57 |
| North East and Yorkshire | 0.45 | 0.36, 0.55 |
| North West | 1.67 | 1.41, 2.01 |
| Southern and Eastern | 0.37 | 0.31, 0.44 |
| Gender | ||
| Male | 1.00 | |
| Female | 1.50 | 1.31, 1.73 |
| Injecting duration, year(s) | ||
| <1 | 1.00 | |
| 1 | 1.27 | 1.23, 1.34 |
| 5 | 2.84 | 2.47, 3.38 |
| 10 | 6.84 | 4.99, 9.99 |
| 15 | 9.11 | 7.39, 11.80 |
| Age, years | ||
| 20 | 1.00 | |
| 30 | 1.63 | 1.44, 1.85 |
| 40 | 2.33 | 1.98, 2.76 |
| 50 | 2.90 | 2.23, 3.91 |
Odds ratios are presented for the mean characteristics of the study population.
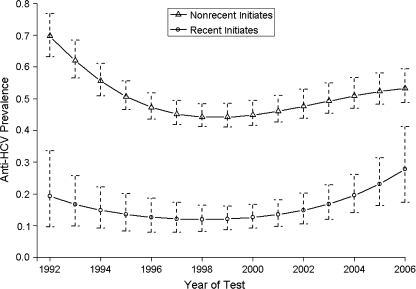
Figure 2 shows the estimated prevalence trends from the model in which individuals are classified as recent and nonrecent initiates. Results reveal a comparatively high HCV adjusted prevalence in individuals who have injected for less than 1 year—at 19% (95% credible interval: 10, 34) in 1992 approximately halving by 1998 and then increasing to 28% (95% credible interval: 17, 41) in 2006. The increase from 1998 has been more marked in recent than nonrecent initiates. The probability that HCV prevalence is higher in 2006 than in 1992 for recent initiates is estimated as 83.9%.
Figure 2.
Estimated hepatitis C virus (HCV) antibody prevalence trend for injecting drug users by recent and nonrecent initiates, England and Wales, 1992–2006. All other covariates are set to their mean values. Error bars show 95% credible intervals.
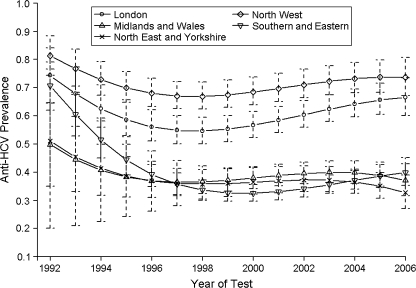
Strong regional effects are identified, and Figure 3 shows the estimated prevalence trend by geographic region. HCV prevalence in London and the North West is markedly higher than in the other regions and, by 2006, had risen on average to 70%. In London, there is an 8.3% probability that prevalence in 2006 is as high as it was in 1992. The trends from the other regions all overlap, with the estimated prevalence especially uncertain in the early years (because of small samples from some areas), and in general suggest that HCV risk fell in the early 1990s and from 1998 flattened out. Analyses that further subdivided the regions in the rest of England and Wales (without London and the North West region) gave a similar picture but also increased the uncertainty surrounding the average estimates (results not shown).
Figure 3.
Estimated hepatitis C virus (HCV) antibody prevalence trend for injecting drug users by region, England and Wales, 1992–2006. All other covariates are set to their mean values. Error bars show 95% credible intervals for each region.
The effect of age on the risk of HCV infection is presented in Table 1 for selected ages. Despite adjustment for injecting duration, so that at each age the average duration is the same, there is still a clear age effect, with older individuals having a higher risk of infection than younger IDUs.
DISCUSSION
Our analysis of public health surveillance data suggests that, in England and Wales, HCV risk prevalence among IDUs declined during the 1990s but that the trend reversed after 1998 such that, in 2006 in some areas and among some subgroups of IDUs, HCV prevalence was approaching or even greater than that in 1992. In addition, we suggest that, in England and Wales, marked geographic differences in HCV prevalence remain, and prevalence among recent injectors of <1 year has increased rapidly since 1998 and is higher than the HCV prevalence in 1992. This is consistent with results from some studies in the United Kingdom that suggest increased HCV prevalence among recent initiates (25, 26) and that any change in HCV risk would occur more quickly among recent injectors. In our models, the risk of infection was estimated to increase monotonically with injecting duration, after adjustment for age and year of test (i.e., any cohort effect). This is to be expected, because the risk of current or past infection, as measured through anti-HCV antibodies, can only accumulate with exposure.
We found that, even after adjustment (for age, injecting duration, and year of test), women are more likely to be infected than men, and older injectors with the same average injecting duration are more likely to be infected than younger injectors. In contrast, a recent meta-analysis of HCV prevalence studies reported no significant difference in prevalence between men and women (27). The difference in HCV prevalence risk experienced by older injectors may be due to residual cohort effects that have not fully been accounted for or, alternatively, may reflect differential exposure to other injectors with a higher risk of HCV. Differential exposure could also explain why we found a higher adjusted prevalence in females. That is, women may tend to inject with older men with a longer injecting duration and therefore are at greater risk of exposure to HCV. Such a pattern has been observed in a network of New York IDUs (28). An alternative hypothesis is that women also may be more vulnerable and may be less able to control their injecting environment and risk than men (29–31). Equally, people who start injecting at an older age (after 25 years) may have greater comorbidity and/or other risks, such as homelessness and prison history, that increase their HCV risk, or there may be a greater misclassification of injecting duration among older injectors. These hypotheses and explanations need to be explored in future research and targeted prevention.
Strengths and limitations
Two key strengths of this work are the sources of data and analytical approach. First, the data were obtained from an ongoing surveillance program that has recruited reasonably sized (n ≈ 2,000) annual samples by using the same methodology over time, although the service provision from which samples are drawn has necessarily changed over the time period. Second, we deployed innovative statistical methodology to adjust for changes in the accuracy of the testing device by allowing uncertainty in the knowledge of the level of sensitivity and specificity in the adjustment process. We also modeled age and injecting duration as continuous variables using polynomials. This approach provides a better fit to the relation between age/duration of injecting and HCV risk, which is not linear, and reduces the number of parameters compared with introducing categorical data (16).
The resulting estimated HCV prevalence overall and among subgroups is based on the average characteristics of the sample, and therefore the emphasis of this work is on relative changes in HCV risk over time, which we feel are more readily interpretable and the appropriate focus of routine surveillance. However, the estimates do not provide direct information on the true HCV prevalence in the IDU population, which can be addressed only if we assumed that we had recruited a representative sample of IDUs or had external information to generate specific sample weights that could be applied to the routine surveillance data. Such an assumption is not warranted, and data for the latter are unavailable.
We acknowledge several related limitations. First, although we sampled from similar sites (n ≈ 50 low threshold treatment and syringe exchanges) each year, these have changed over time broadly reflecting changes in service provision. The changes in distribution and types of services provided will, in part, reflect changing patterns of drug use and the types of drug being used, as well as developments in drug policy, such as expanded provision of specialist drug treatment. Although we adjusted for several potential confounders, it is possible that the case-mix and other unmeasured factors (such as severity of dependence, proportion of former prisoners, and quicker access to treatment) relating to HCV risk have also changed over time.
Second, we lacked power and continuous surveillance data to investigate a more refined classification of geographic areas. There are cities in the defined geographic regions (such as Bristol and Brighton in the Southern and Eastern region and Leeds in the North East and Yorkshire region) that have higher levels of HCV than other cities, towns, and rural areas in these regions (16). Preliminary investigations suggested that, in the Southern and Eastern region, the proportion of high prevalence sampling sites did not remain constant over time. However, further analyses subdividing this region into “high,” “medium,” and “low” prevalence sites found a generally consistent overall picture of trend (data not shown).
Third, we do not attempt to explain the differences in HCV prevalence, for example, by fitting additional covariates on injecting risk behavior or the availability and intensity of prevention services. In part, this is because full data on risk behavior and other predictors are unavailable, but primarily because the main purpose of our analyses was to provide basic public health evidence on whether HCV risk (as measured by estimated anti-HCV prevalence) has changed over time.
Our work attempts to estimate trends in HCV prevalence and models prevalence in recent (<1 year) injectors as a measure of incidence, but another possible approach would be to estimate incidence directly from serial prevalence and exposure information (e.g., refer to references 32 and 33). These approaches directly address issues concerning incidence and transmission. Nevertheless, results from our model still provide useful information concerning the underlying epidemic among IDUs.
Interpretation—public health intelligence
There are 2 key messages for policy makers and practitioners in England and Wales and elsewhere. First, HCV among IDUs can be controlled: There are some places with low HCV prevalence and HCV risk fell during the early to mid-1990s, even in sites with a high background prevalence of HCV. The declines in risk support the hypothesis that the health education, needle exchange, and opiate substitution treatment widely used in the United Kingdom in the late 1980s changed behavior and injecting risk for HCV as well as HIV infection among IDUs (34–36).
Second, increased action and prevention services are required. Since 1998, HCV risk has remained stable or increased and, among some IDUs (particularly recent injectors), any benefits of the risk reduction achieved in earlier years have been lost. There are several potential and overlapping reasons for the increase in risk that cannot be explored by these data alone. These include increases in the prevalence of injecting over and above any increase in prevention services (37, 38) and/or changes in the characteristics of the IDU population (such as increased homelessness and crack-cocaine injection) that may have led to increased injecting risk (16). Whatever the explanation, and it is likely to be a combination of reasons, the key public health challenge is to reverse the currently rising HCV risk through increasing effective intervention coverage (8, 39, 40). The challenge to our public health surveillance program is to improve information collected on the characteristics of IDUs so that we can develop better models of HCV risk in order to inform and measure the impact of public health action.
Acknowledgments
Author affiliations: Medical Research Council Biostatistics Unit, Institute of Public Health, Cambridge, United Kingdom (Michael J. Sweeting, Daniela De Angelis); Centre for Research on Drugs and Health Behaviour, Department of Public Health and Policy, London School of Hygiene and Tropical Medicine, London, United Kingdom (Vivian D. Hope); Centre for Infections, Health Protection Agency, London, United Kingdom (Vivian D. Hope, John V. Parry, Fortune Ncube, Mary E. Ramsay, Daniela De Angelis); and Department of Social Medicine, University of Bristol, Bristol, United Kingdom (Matthew Hickman).
This work was supported by the United Kingdom Department of Health that financed the retrospective testing of stored sera and provided funding to M. J. S. (AIDB 2/29).
The authors would like to thank all the collaborators involved with the unlinked anonymous prevalence monitoring program, as well as the drug users who gave their time to be interviewed.
Conflict of interest: none declared.
Glossary
Abbreviations
- AIC
Akaike Information Criterion
- CI
confidence interval
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IDU
injecting drug user
Appendix Table 1.
Observed Hepatitis C Virus Antibody Prevalence by Covariate Level, England and Wales, 1992–2006
| Variable | No. Positive | No. Tested | Proportion Positive |
| Year of test | |||
| 1992 | 850 | 1,800 | 0.47 |
| 1994 | 868 | 2,134 | 0.41 |
| 1996 | 640 | 1,810 | 0.35 |
| 1998 | 896 | 2,306 | 0.39 |
| 1999 | 830 | 2,485 | 0.33 |
| 2000 | 791 | 2,362 | 0.33 |
| 2001 | 716 | 2,063 | 0.35 |
| 2002 | 741 | 1,819 | 0.41 |
| 2003 | 743 | 1,693 | 0.44 |
| 2004 | 704 | 1,572 | 0.45 |
| 2005 | 824 | 1,828 | 0.45 |
| 2006 | 824 | 1,935 | 0.43 |
| Sex | |||
| Male | 7,196 | 18,162 | 0.40 |
| Female | 2,171 | 5,510 | 0.39 |
| Region | |||
| London | 2,329 | 4,418 | 0.53 |
| Midlands and Wales | 865 | 2,868 | 0.30 |
| North East and Yorkshire | 611 | 2,999 | 0.20 |
| North West | 3,254 | 6,089 | 0.53 |
| Southern and Eastern | 2,368 | 7,431 | 0.32 |
| Injecting duration, years | |||
| 0–4 | 1,619 | 7,897 | 0.21 |
| 5–9 | 2,201 | 6,077 | 0.36 |
| 10–14 | 2,008 | 4,032 | 0.50 |
| ≥15 | 3,119 | 4,755 | 0.66 |
| Age, years | |||
| 15–24 | 1,155 | 5,773 | 0.20 |
| 25–29 | 2,071 | 6,100 | 0.34 |
| 30–34 | 2,381 | 5,331 | 0.45 |
| 35–59 | 3,600 | 6,158 | 0.58 |
Appendix Table 2.
Parameter Estimates From a Classical Logistic Regression Model With Lowest AIC Statistic for Injecting Drug Users, England and Wales, 1992–2006
| Parameter | Estimate | Standard Error | z Value | P Value |
| Intercept | −0.105 | 0.058 | −1.82 | 0.069 |
| Year of test | ||||
| Linear | 0.081 | 0.012 | 6.57 | <0.001 |
| Quadratic | 0.0045 | 0.0022 | 2.05 | 0.041 |
| Cubic | −0.00112 | 0.00026 | −4.38 | <0.001 |
| Age, years | ||||
| Linear | 0.0124 | 0.0066 | 1.87 | 0.061 |
| Quadratic | −0.00069 | 0.00027 | −2.52 | 0.012 |
| Gender, females | 0.262 | 0.085 | 3.07 | 0.002 |
| Injecting duration, years | ||||
| Linear | 0.1131 | 0.0072 | 15.79 | <0.001 |
| Quadratic | −0.00394 | 0.00061 | −6.48 | <0.001 |
| Cubic | 0.000061 | 0.000025 | 2.43 | 0.015 |
| Region, baseline London | ||||
| Midlands and Wales | −0.593 | 0.085 | −6.97 | <0.001 |
| North East and Yorkshire | −0.713 | 0.095 | −7.54 | <0.001 |
| North West | 0.404 | 0.069 | 5.83 | <0.001 |
| Southern and Eastern | −0.876 | 0.070 | −12.42 | <0.001 |
| Gender × injecting duration | ||||
| Females × linear effect | −0.0169 | 0.0080 | −2.13 | 0.033 |
| Females × quadratic effect | −0.0036 | 0.0012 | −3.12 | 0.002 |
| Females × cubic effect | 0.000190 | 0.000063 | 2.99 | 0.003 |
| Injecting duration × region | ||||
| Linear × Midlands and Wales | −0.014 | 0.010 | −1.35 | 0.178 |
| Linear × North East and Yorkshire | 0.039 | 0.013 | 2.97 | 0.003 |
| Linear × North West | −0.0230 | 0.0082 | −2.79 | 0.005 |
| Linear × Southern and Eastern | 0.0064 | 0.0078 | 0.83 | 0.409 |
| Year of test × injecting duration | ||||
| Linear × linear | −0.00142 | 0.00075 | −1.91 | 0.057 |
| Quadratic × linear | −0.00084 | 0.00017 | −5.02 | <0.001 |
| Linear × quadratic | 0.000110 | 0.000061 | 1.82 | 0.069 |
| Quadratic × quadratic | 0.000028 | 0.000014 | 1.98 | 0.048 |
| Year of test × region | ||||
| Linear × Midlands and Wales | −0.039 | 0.015 | −2.63 | 0.009 |
| Linear × North East and Yorkshire | −0.057 | 0.025 | −2.29 | 0.022 |
| Linear × North West | −0.000 | 0.012 | −0.00 | 0.999 |
| Linear × Southern and Eastern | −0.055 | 0.011 | −4.91 | <0.001 |
| Quadratic × Midlands and Wales | −0.0045 | 0.0033 | −1.35 | 0.177 |
| Quadratic × North East and Yorkshire | −0.0043 | 0.0052 | −0.82 | 0.413 |
| Quadratic × North West | −0.0036 | 0.0026 | −1.39 | 0.164 |
| Quadratic × Southern and Eastern | 0.0046 | 0.0026 | 1.75 | 0.079 |
| Age × region | ||||
| Linear × Midlands and Wales | 0.025 | 0.011 | 2.40 | 0.017 |
| Linear × North East and Yorkshire | −0.002 | 0.012 | −0.20 | 0.841 |
| Linear × North West | 0.0268 | 0.0084 | 3.17 | 0.002 |
| Linear × Southern and Eastern | 0.0381 | 0.0082 | 4.63 | <0.001 |
| Gender × region | ||||
| Females × Midlands and Wales | −0.16 | 0.13 | −1.19 | 0.233 |
| Females × North East and Yorkshire | 0.04 | 0.15 | 0.28 | 0.777 |
| Females × North West | −0.01 | 0.10 | −0.13 | 0.894 |
| Females × Southern and Eastern | 0.25 | 0.10 | 2.49 | 0.013 |
Abbreviation: AIC, Akaike Information Criterion.
References
- 1.Alter MJ, Moyer LA. The importance of preventing hepatitis C virus infection among injection drug users in the United States. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(suppl 1):S6–S10. doi: 10.1097/00042560-199802001-00003. [DOI] [PubMed] [Google Scholar]
- 2.Roy K, Hay G, Andragetti R, et al. Monitoring hepatitis C virus infection among injecting drug users in the European Union: a review of the literature. Epidemiol Infect. 2002;129(3):577–585. doi: 10.1017/s0950268802007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dore GJ, Law M, MacDonald M, et al. Epidemiology of hepatitis C virus infection in Australia. J Clin Virol. 2003;26(2):171–184. doi: 10.1016/s1386-6532(02)00116-6. [DOI] [PubMed] [Google Scholar]
- 4.Hagan H, Pouget ER, Des Jarlais DC, et al. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168(10):1099–1109. doi: 10.1093/aje/kwn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Angelis D, Sweeting M, Ades AE, et al. An evidence synthesis approach to estimating hepatitis C prevalence in England and Wales. Stat Methods Med Res. doi: 10.1177/0962280208094691. Advance Access: November 26, 2008. (DOI: 10.1177/0962280208094691) [DOI] [PubMed] [Google Scholar]
- 6.Sweeting MJ, De Angelis D, Ades AE, et al. Estimating the prevalence of ex-injecting drug use in the population. Stat Methods Med Res. doi: 10.1177/0962280208094704. Advance Access: November 26, 2008. (DOI: 10.1177/0962280208094704) [DOI] [PubMed] [Google Scholar]
- 7.Tilson H, Aramrattana A, Bozzette SA, et al. Preventing HIV Infection Among Injecting Drug Users in High-Risk Countries: An Assessment of the Evidence. Washington, DC: Institute of Medicine; 2007. [Google Scholar]
- 8.Advisory Council on Misuse of Drugs. The Primary Prevention of Hepatitis C Among Injecting Drug Users. London, United Kingdom: Home Office; 2009. [Google Scholar]
- 9.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–199. [Google Scholar]
- 10.Falster K, Kaldor JM, Maher L, et al. Hepatitis C virus acquisition among injecting drug users: a cohort analysis of a national repeated cross-sectional survey of needle and syringe program attendees in Australia, 1995–2004. J Urban Health. 2009;86(1):106–118. doi: 10.1007/s11524-008-9330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson SJ, McIntyre PG, Molyneaux P, et al. Prevalence of hepatitis C among injectors in Scotland 1989–2000: declining trends among young injectors halt in the late 1990s. Epidemiol Infect. 2002;128(3):473–477. doi: 10.1017/s0950268802006945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amon JJ, Garfein RS, Ahdieh-Grant L, et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994–2004. Clin Infect Dis. 2008;46(12):1852–1858. doi: 10.1086/588297. [DOI] [PubMed] [Google Scholar]
- 13.Des Jarlais DC, Perlis T, Arasteh K, et al. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001. AIDS. 2005;19(suppl 3):S20–S25. doi: 10.1097/01.aids.0000192066.86410.8c. [DOI] [PubMed] [Google Scholar]
- 14.Hope VD, Judd A, Hickman M, et al. HIV prevalence among injecting drug users in England and Wales 1990 to 2003: evidence for increased transmission in recent years. AIDS. 2005;19(11):1207–1214. doi: 10.1097/01.aids.0000176222.71355.a1. [DOI] [PubMed] [Google Scholar]
- 15.Noone A, Durante AJ, Brady AR, et al. HIV infection in injecting drug users attending centres in England and Wales, 1990–1991. AIDS. 1993;7(11):1501–1507. doi: 10.1097/00002030-199311000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Hickman M, Hope V, Brady T, et al. Hepatitis C virus (HCV) prevalence, and injecting risk behaviour in multiple sites in England in 2004. J Viral Hepat. 2007;14(9):645–652. doi: 10.1111/j.1365-2893.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 17.Hickman M, Higgins V, Hope V, et al. Injecting drug use in Brighton, Liverpool, and London: best estimates of prevalence and coverage of public health indicators. J Epidemiol Community Health. 2004;58(9):766–771. doi: 10.1136/jech.2003.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Judd A, Parry J, Hickman M, et al. Evaluation of a modified commercial assay in detecting antibody to hepatitis C virus in oral fluids and dried blood spots. J Med Virol. 2003;71(1):49–55. doi: 10.1002/jmv.10463. [DOI] [PubMed] [Google Scholar]
- 19.le Cessie S, van Houwelingen JC. A goodness-of-fit test for binary regression models, based on smoothing methods. Biometrics. 1991;47(4):1267–1282. [Google Scholar]
- 20.Van Buuren S, Oudshoorn CGM. Flexible Multivariate Imputation by MICE. Leiden, the Netherlands: TNO Prevention and Health; 1999. ( http://web.inter.nl.net/users/S.van.Buuren/mi/docs/rapport99054.pdf). (Accessed April 24, 2009) [Google Scholar]
- 21.Magder LS, Hughes JP. Logistic regression when the outcome is measured with uncertainty. Am J Epidemiol. 1997;146(2):195–203. doi: 10.1093/oxfordjournals.aje.a009251. [DOI] [PubMed] [Google Scholar]
- 22.McInturff P, Johnson WO, Cowling D, et al. Modelling risk when binary outcomes are subject to error. Stat Med. 2004;23(7):1095–1109. doi: 10.1002/sim.1656. [DOI] [PubMed] [Google Scholar]
- 23.Spiegelhalter D, Thomas A, Best N, et al. WinBUGS Version 1.4 User Manual. Cambridge, United Kingdom: MRC Biostatistics Unit; 2003. [Google Scholar]
- 24.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7(4):434–455. [Google Scholar]
- 25.Judd A, Hickman M, Jones S, et al. Incidence of hepatitis C virus and HIV among new injecting drug users in London: prospective cohort study. BMJ. 2005;330(7481):24–25. doi: 10.1136/bmj.38286.841227.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutton AJ, Hope VD, Mathei C, et al. A comparison between the force of infection estimates for blood-borne viruses in injecting drug user populations across the European Union: a modelling study. J Viral Hepat. 2008;15(11):809–816. doi: 10.1111/j.1365-2893.2008.01041.x. [DOI] [PubMed] [Google Scholar]
- 27.Hagan H, Des Jarlais DC, Stern R, et al. HCV synthesis project: preliminary analyses of HCV prevalence in relation to age and duration of injection. Int J Drug Policy. 2007;18(5):341–351. doi: 10.1016/j.drugpo.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Friedman SR, Curtis R, Neagius A, et al. Social Networks, Drug Injectors’ Lives, and HIV/AIDS. New York, NY: Kluwer/Plenum; 1999. [Google Scholar]
- 29.Sheard L, Tompkins C. Contradictions and misperceptions: an exploration of injecting practice, cleanliness, risk, and partnership in the lives of women drug users. Qual Health Res. 2008;18(11):1536–1547. doi: 10.1177/1049732308325838. [DOI] [PubMed] [Google Scholar]
- 30.Breen C, Roxburgh A, Degenhardt L. Gender differences among regular injecting drug users in Sydney, Australia, 1996–2003. Drug Alcohol Rev. 2005;24(4):353–358. doi: 10.1080/09595230500263871. [DOI] [PubMed] [Google Scholar]
- 31.Evans JL, Hahn JA, Page-Shafer K, et al. Gender differences in sexual and injection risk behavior among active young injection drug users in San Francisco (the UFO Study) J Urban Health. 2003;80(1):137–146. doi: 10.1093/jurban/jtg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacchetti P, Tien P, Seaberg E, et al. Estimating past hepatitis C infection risk from reported risk factor histories: implications for imputing age of infection and modeling fibrosis progression [electronic article] BMC Infect Dis. 2007;7:145. doi: 10.1186/1471-2334-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ades AE, Nokes DJ. Modeling age- and time-specific incidence from seroprevalence: toxoplasmosis. Am J Epidemiol. 1993;137(9):1022–1034. doi: 10.1093/oxfordjournals.aje.a116758. [DOI] [PubMed] [Google Scholar]
- 34.Hope VD, Judd A, Hickman M, et al. Prevalence of hepatitis C among injection drug users in England and Wales: is harm reduction working? Am J Public Health. 2001;91(1):38–42. doi: 10.2105/ajph.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madden PB, Lamagni T, Hope V, et al. The HIV epidemic in injecting drug users. Commun Dis Rep CDR Rev. 1997;7(9):R128–R130. [PubMed] [Google Scholar]
- 36.Stimson GV. AIDS and injecting drug use in the United Kingdom, 1987–1993: the policy response and the prevention of the epidemic. Soc Sci Med. 1995;41(5):699–716. doi: 10.1016/0277-9536(94)00435-v. [DOI] [PubMed] [Google Scholar]
- 37.Morgan O, Griffiths C, Hickman M. Association between availability of heroin and methadone and fatal poisoning in England and Wales 1993–2004. Int J Epidemiol. 2006;35(6):1579–1585. doi: 10.1093/ije/dyl207. [DOI] [PubMed] [Google Scholar]
- 38.Morgan O, Vicente J, Griffiths P, et al. Trends in overdose deaths from drug misuse in Europe: what do the data tell us? Addiction. 2008;103(5):699–700. doi: 10.1111/j.1360-0443.2007.02102.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Den Berg C, Smit C, Van Brussel G, et al. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102(9):1454–1462. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Den Berg CH, Smit C, Bakker M, et al. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. Eur J Epidemiol. 2007;22(3):183–193. doi: 10.1007/s10654-006-9089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]