Abstract
Whether the main energy source for sperm motility is from oxidative phosphorylation or glycolysis has been long-debated in the field of reproductive biology. Using the rhesus monkey as a model, we examined the role of glycolysis and oxidative phosphorylation in sperm function by using alpha-chlorohydrin (ACH), a glycolysis inhibitor, and pentachlorophenol (PCP), an oxidative phosphorylation uncoupler. Sperm treated with ACH showed no change in percentage of motile sperm, although sperm motion was impaired. The ACH-treated sperm did not display either hyperactivity- or hyperactivation-associated changes in protein tyrosine phosphorylation. When treated with PCP, sperm motion parameters were affected by the highest level of PCP (200 μM); however, PCP did not cause motility impairments even after chemical activation. Sperm treated with PCP were able to display hyperactivity and tyrosine phosphorylation after chemical activation. In contrast with motility measurements, treatment with either the glycolytic inhibitor or the oxidative phosphorylation inhibitor did not affect sperm-zona binding and zona-induced acrosome reaction. The results suggest glycolysis is essential to support sperm motility, hyperactivity, and protein tyrosine phosphorylation, while energy from oxidative phosphorylation is not necessary for hyperactivated sperm motility, tyrosine phosphorylation, sperm-zona binding, and acrosome reaction in the rhesus macaque.
Keywords: acrosome reaction, sperm, sperm capacitation
Energy from glycolysis but not oxidative phosphorylation is essential to support sperm motility, hyperactivated motility, and protein tyrosine phosphorylation in rhesus macaque.
INTRODUCTION
Mammalian spermatozoa are highly specialized cells. One of the distinct features of sperm is motility generated by the elongated flagellum. The motility of sperm is essential to transport the genetic materials to the site of fertilization. The percentage of motile sperm is used as one of the major indices in fertility clinics to determine whether an individual man is fertile. The source of ATP in support of sperm motility has been long debated in the field of gamete research. The two major mechanisms that have been considered for energy production in spermatozoa are oxidative phosphorylation from the mitochondria and glycolysis in the principal piece of the flagellum. Both of these possibilities have been investigated many times, but the results remain inconclusive.
The mitochondria are located in the midpiece of the flagellum and have been thought to supply the energy to drive the flagellum. In support of mitochondrial involvement in sperm motility, studies have shown that bull sperm motility is correlated with oxygen consumption [1]. Moreover, men with mitochondrial DNA mutations related to oxidative phosphorylation have reduced sperm motility [2]. Bull sperm were able to sustain motility under aerobic condition when glycolysis was inhibited [3], and inhibition of respiratory chain of ATP synthesis in the mitochondria by an uncoupling agent decreased sperm motility in bovine samples [4, 5]. An early study has also shown that human sperm can obtain energy from oxidative phosphorylation, and because of its high efficiency, the net yield of ATP might play an important role in sperm physiology [6]. However, whether the ATP produced by the mitochondria can be delivered in sufficient quantities to supply the entire flagellum is still under debate [7–9].
Glycolytic enzymes are located mainly in the principal piece and in the fibrous sheath of the flagellum in a variety of mammalian species including rabbits, mice, and foxes [10–12]. A recent study used a proteomic approach to identify a sperm flagellar energy carrier protein along with seven glycolytic enzymes in the fibrous sheath in the principal piece of human sperm [13]. These findings support the theory that the ATP produced adjacent to the site of energy requirement can effectively support the demand. Glycolysis plays a significant role in the energy production in boar sperm [14]. In stable isotope and radiotracer studies with glucose, boar sperm readily converted glucose to glucose-6-phosphate and lactate via glycolysis with no involvement of pentose phosphate metabolism [14]. Another study showed that mice with a gene knockout for the sperm specific isoform of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) have immotile sperm, which supports the importance of glycolysis in sperm motility [15]. In addition to its role in sperm motility, in some studies glucose was found to be involved in sperm capacitation in mice since ATP produced by glycolytic pathways was sufficient to support capacitation associated sperm tyrosine phosphorylation. [16, 17]. However, other studies showed that ram and mouse sperm treated with the GAPDH inhibitor, α-chlorohydrin (ACH), can maintain motility and ATP concentration for at least 1 h without external glucose, indicating that glycolysis is not required to support normal motility [18, 19]. Another study also showed that when mouse sperm were incubated in a medium without glucose for 90 min, the percentage of sperm showing capacitation and progressive motility as well as values for curvilinear velocity and linearity were not different from the sperm in medium with glucose [20]. Such species differences suggest that mechanisms underlying sperm ATP production and sources may differ between species.
Previous studies have been mostly focused on whether oxidative phosphorylation or glycolysis supports sperm motility by measuring the consumption of glycolysis substrates or oxygen for oxidative phosphorylation. In the present study, we used specific inhibitors to these processes to examine whether glycolysis or oxidative phosphorylation is the major energy source supporting sperm motility in rhesus monkey sperm. ACH, which inhibits the glycolytic enzymes GAPDHs and triosephosphate isomerase (TPI) in sperm to prevent ATP production from glycolysis, was a potential male contraceptive until its epididymal toxicity as well as neurotoxic effect and bone marrow damage were found [21–25]. Pentachlorophenol (PCP) is an oxidative phosphorylation inhibitor and has been used to study sperm motility in other species [26, 27]. With these chemicals, the importance of glycolysis or oxidative phosphorylation can be more easily assessed. In addition to sperm motility, capacitation, sperm-zona binding, and zona-induced acrosome reaction were also examined to provide more information about the role of glycolysis and oxidative phosphorylation in rhesus monkey sperm physiology.
MATERIALS AND METHODS
Sperm Collection and Washing
Three fertile adult male rhesus macaques were caged individually at the California National Primate Research Center in compliance with the Federal Animal Welfare Act and the NIH Guidelines for Care and Use of Laboratory Animals. The animals were maintained on a 12L:12D light cycle (lights on at 0600 h) at 25∼27°C and were given a diet of Purina monkey chow and water ad libitum. The males were trained to chair restraint and were electroejaculated with a Grass 6 stimulator (Grass Medical Instruments, Quincy, MA) equipped with EKG pad electrodes (Conmed Corp., Utica, NY) for direct penile stimulation (30∼50 V, 20-msec duration, 18 pulse/sec) [28]. Semen samples, collected in 50-ml centrifuge tubes, were allowed to stand for 30 min at 22°C before the coagulum was removed. Each semen sample was processed separately. Semen was transferred to a 15-ml tube and washed three times for 10 min at 300 × g with 15 ml Tyrode medium (TL-BSA) that was modified and supplemented with 3 mg/ml bovine serum albumin and lactate [29] and resuspended in media described later. For some experiments, semen was split into two 15-ml tubes. One was washed with TL-BSA containing glucose, and the other was washed with TL-BSA without glucose (TL-BSA-NG).
Experimental Design
Sperm were suspended in TL-BSA (with or without glucose), with ACH (0.1∼5 mM with or without glucose), or pentochlorophenol (PCP, 50∼200 μM) at a concentration of 10 × 106 sperm per milliliter. Sperm concentration was determined with a hemocytometer. Sperm from each male were treated with different chemicals (randomized complete block design) and were incubated for 1 h at 37°C in 5% CO2 before assessment. Sperm were evaluated immediately after incubation for motility, CASA analysis, and mitochondrial integrity. Sperm were then activated by incubating with 1 mM dbcAMP (Alexis Chemicals, San Diego, CA) and 1mM caffeine (Sigma Chemical Co., St. Louis, MO) for an additional 30 min [30]. Further sperm motility, CASA analysis, and sperm-zona binding assay were performed.
Assessment of Percentage of Motile Sperm
A sperm was considered motile when it displayed any movement in this study. The percentage of motile sperm (% motility) was determined by visual observation at 200× magnification of 100 sperm using an Olympus BH2 microscope (Scientific Instruments Co., Sunnyvale, CA). Prior to treatment, semen samples used in these experiments had greater than 90% motile sperm, of which 90% had forward progression. After 1 h of treatment and after 30 min of activation, a 10-μl aliquot of sperm suspension was placed onto a slide and overlaid with a 22-mm2 cover slip, and the slide was maintained at 37°C during analysis by a heated stage, and the percentage of motile sperm was evaluated [30].
CASA Assessment of Sperm Motion Parameters
After either 1 h of treatment or 1 h of treatment followed by 30 min of activation, a 10-μl aliquot of sperm suspension was placed onto a microscope slide and overlaid with a 22-mm2 cover slip, and the slide was maintained at 37°C during analysis by a heated stage. Sperm motility of at least 10 fields at 37°C was recorded onto videotape using a VHS recorder (Panasonic Co., Secaucus, NJ) utilizing a CCD video camera (MTI Technology Co., Tustin, CA) mounted on an Olympus BH2 microscopy. A dark-field 4× objective was used, and the total magnification for videotaping was 40. The VHS tape video was transformed to a digital videocassette for analysis. At least 200 cells per sample in a minimum of four fields were analyzed by computer-automated sperm analysis (CASA) utilizing HTM Ceros (Version 12.2 g; Hamilton-Thorne Research, Beverly, MA). Instrument settings for the CASA analysis were as follows: frame rate 60 Hz, frames acquired 30, minimum contrast 80, minimum cell size four pixels, static VAP cutoff 20 μ/sec, static VSL cutoff 10 μ/sec, progressive VAP threshold 25 μ/sec, progressive STR threshold 80%, static intensity limits 0.6–1.4, static size limits 0.6–2.31, and static elongation limits 0–80. Average path velocity (VAP), straight-line velocity (VSL), curvilinear velocity (VCL), linearity (LIN), amplitude of lateral head displacement (ALH), beat cross frequency (BCF), straightness (STR), and percentage of hyperactivated sperm (HYP) were determined. The threshold to determine the hyperactivated sperm is for VCL ≥ 130 μm/sec, ALH ≥ 7.5 Hz, and LIN ≤ 69% [31, 32].
Evaluation of Mitochondrial Integrity and Changes of Membrane Potential
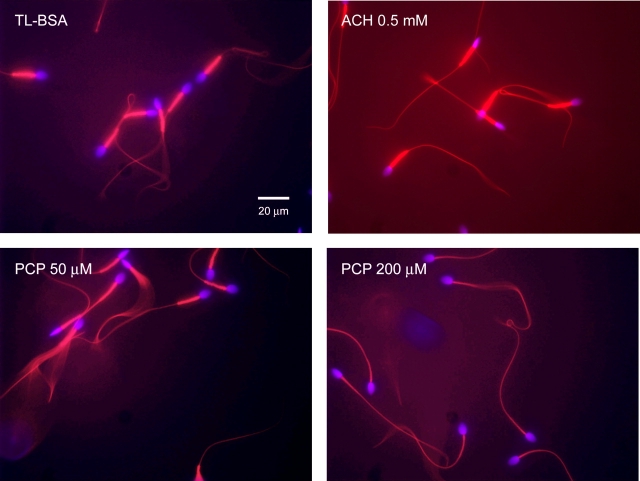
Live mitochondria were labeled with 200 nM MitoTracker Red CMXRos (Molecular Probes, Eugene, OR) for 30 min after 1 h of ACH or PCP treatment in glucose-containing media. A 10-μl sample of each sperm suspension was placed onto a glass slide and mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Sperm images were taken with Provis Production Microscopy at California National Primate Research Center. With the same field, DAPI and MitoTracker Red images were taken by using a 40× objective lens. The resolution of each image was set to 300 dpi. The DAPI and MitoTracker Red images were combined to create one fluorescent image. Sperm mitochondria showing red fluorescent labeling were intact. Sperm that underwent several freeze–thaw cycles served as negative control.
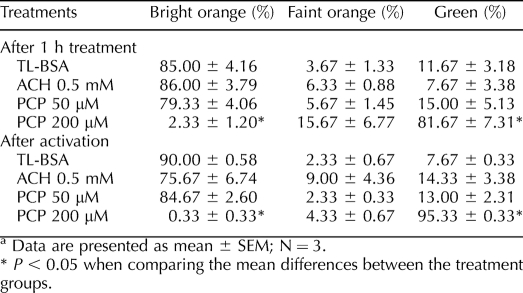
To evaluate the changes of mitochondrial membrane potential, sperm were labeled with potential-sensitive 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1; Molecular Probes) for 10 min after 1 h of ACH or PCP treatment, and after 30 min of chemical activation. Sperm then were fixed with 50 μl 4% formaldehyde, and the supernatant was removed and the sperm were resuspended in DPBS. The percentage of sperm with green (inactive mitochondria, no membrane potential), bright orange (active mitochondria, high membrane potential), or faint orange staining was evaluated visually by using Olympus BH2 fluorescence microscopy, and a total of 100 sperm were scored in each treatment.
Measurement of Intracellular ATP
After 1 h incubation with ACH or PCP, sperm protein was precipitated by addition of ice-cold 100% ethanol (1:3.3 dilution, final 70%). The samples were vortexed, ultrasonically homogenized, and dried by liquid nitrogen. The samples were resuspended in dH2O, and ATP was measured by the luminometric methods as previously described [33] by using Adenosine 5′-triphosphate (ATP) Bioluminescent Assay Kit (Sigma) on a Turner Luminometer (TE20).
Sperm-Zona Binding and Zona Pellucida-Induced Acrosome Reaction
Sperm-zona binding experiments were performed as previously described [30]. Briefly, sperm were activated by incubating with 1 mM dbcAMP (Alexis Chemicals) and 1 mM caffeine (Sigma) for 30 min. Then, two to four oocytes were placed in 100-μl drops of sperm suspension under silicone oil (Aldrich Chemical Co., Milwaukee, WI). After 30 sec of coincubation, the oocytes were quickly rinsed in DPBS and immediately fixed in cold absolute ethanol for 1–2 min. After fixation, oocytes were placed on glass slides, and sperm bound to the zona pellucida were labeled by indirect immunofluorescence using a polyclonal antisperm antiserum produced and employed as described [34]. The number of sperm on the zona pellucida and percentage of acrosomal reacted sperm were evaluated by using Olympus BH2 fluorescence microscopy.
Immunofluorescence Labeling for Protein Tyrosine Phosphorylation
The percentage of sperm with tyrosine phosphorylation was evaluated as described previously [32]. Briefly, sperm were washed three times in DPBS containing 1 mg/ml polyvinyl alcohol (Sigma) after activation, fixed 10 min in 2% paraformadehyde, permeabilized with cold 95% ethanol for 10 min, blocked for 10 min in 5% BSA in DPBS, incubated in 4G10 monoclonal antibody (dilution, 1:500) (Upstate USA, Inc., Charlottesville, VA) at 4°C overnight, and then incubated with fluorescein-conjugated goat anti-mouse (Fab) IgG (Molecular Probes) (dilution, 1:32) for 1 h at room temperature in the dark. One hundred sperm were scored with Olympus BH-2 fluorescence microscopy for the percentage of sperm with tyrosine phosphorylation.
Statistical Analysis
All data are expressed as mean ± SEM. For all data, N represents the number of male monkeys used for each experiment. The data were examined by two-way analysis of variance when comparing the mean differences among treatment groups. Paired t-test was used when comparing the mean differences between pre- and postactivation. A two-sample t-test was used when comparing the mean differences between glucose and no-glucose treatments.
RESULTS
Percentage of Motile Sperm
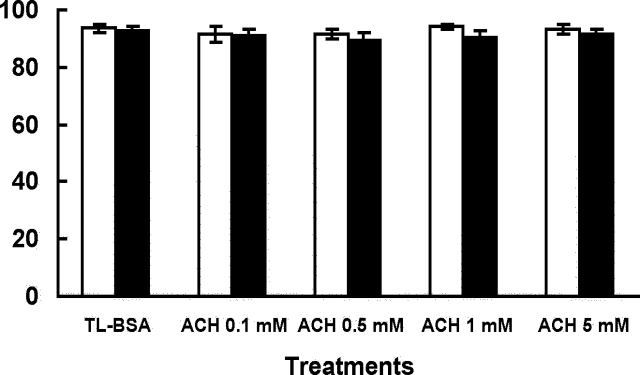
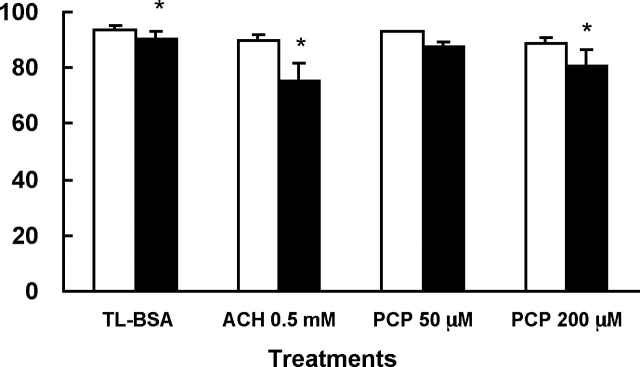
After 1 h of treatment, sperm treated with ACH in glucose or nonglucose media showed no significant difference in the percentage of motile sperm when compared to the TL-BSA control groups (Fig. 1). It should be noted that this manual measurement of percentage motility does not necessarily indicate changes in motion characteristics. However, visually it could be seen that sperm treated with 0.5 mM and 5 mM ACH in glucose-containing media showed slower forward progression when compared to other groups. The 0.5 mM ACH was used treat sperm to compare to the PCP-treated sperm for the rest of the experiments in glucose-containing media. When sperm were treated with ACH or different levels of PCP in glucose-containing media, the percentage of motile sperm did not show any significant change when compared to the TL-BSA control group (Fig. 2). There was no change in percentage motility observed in both PCP treatment groups compared to the control group. Sperm were then chemically activated by dbcAMP and caffeine for 30 min, and the percentage of motile sperm was evaluated. After activation, the percentage of motile sperm decreased in TL-BSA control, ACH 0.5-mM, and PCP 200-μM treatment groups. Although there were changes compared to preactivation in some groups, none of the treatment groups showed significant differences compared to the TL-BSA control group after activation (Fig. 2).
FIG. 1.
Percentage of motile sperm after 1 h of ACH treatment in glucose (open bars) or nonglucose media (closed bars). The percentage of motile sperm after 1 h of incubation showed no difference among groups in both treatments. Data are from four males and presented as mean ± SEM.
FIG. 2.
Percentage of motile sperm after 1 h of ACH or PCP treatment (open bars) and after 30 min of chemical activation (closed bars) in glucose-containing media. The percentage of motile sperm after 1 h of incubation showed no difference among groups. After chemical activation, the ACH 0.5-mM treatment group showed a significant decrease of percentage of motile sperm compared to other treatment groups. Data are from three males and presented as mean ± SEM. *, P < 0.05 when comparing mean differences between preactivation and postactivation.
Sperm Motion Parameters by CASA
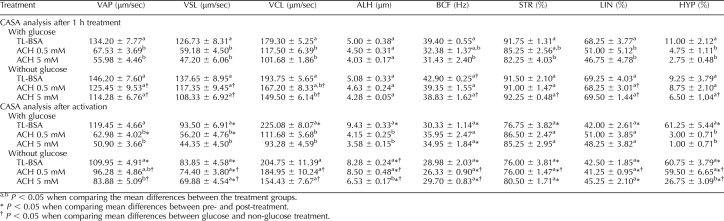
Detailed CASA analysis of sperm motion parameters was carried out after 1 h of treatment and after 1 h of treatment followed by 30 min of incubation with the activators (dbcAMP and caffeine) The CASA data of ACH treated sperm in glucose or nonglucose media are shown in Table 1. Sperm treated with different level of ACH in glucose-containing media for 1 h had dramatically decreased (P < 0.05) VAP, VSL, VCL, LIN, and HYP compared to the TL-BSA control group, while in sperm treated with a different level of ACH in nonglucose media for 1 h, only in the highest ACH level (0.5 mM) was the sperm motion affected (VCL, P < 0.05). After activation, sperm treated with a different level of ACH in glucose-containing media did not show hyperactive motion, while sperm in nonglucose media showed changes only in the 5-mM ACH treatment group (VAP, ALH, and HYP) when compared to the TL-BSA control group.
TABLE 1.
Computer assisted semen analysis of sperm treated with ACH in glucose or non-glucose media (data are presented as mean ± SEM; N = 3).
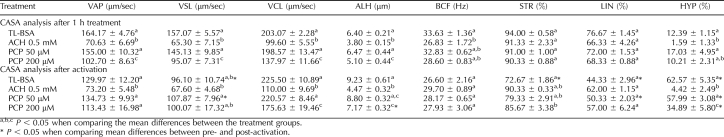
Table 2 depicts a comparison between the effect of ACH and PCP on CASA values in glucose-containing media. Similarly to the data reported in Table 1, sperm treated with 0.5 mM ACH in glucose-containing medium for 1 h had dramatically decreased (P < 0.05) VAP, VSL, VCL, ALH, and BCF compared to the TL-BSA control group (Table 2). The PCP 200-μM treatment group also showed a significant decrease (P < 0.05) of VAP, VSL, VCL, and ALH. The sperm motion parameters of the PCP 50-μM treatment group were similar to those of the TL-BSA control. After activation, the sperm in the TL-BSA control group demonstrated changes consistent with hyperactivity, such as an increase in VCL, ALH, and HYP and a decrease in VSL (P < 0.05), BCF, STR (P < 0.05), and LIN (P < 0.05). The ACH treatment group showed no signs of hyperactivated motility at all, and the sperm motion parameters did not display any changes compared to before activation. The PCP 50-μM treatment group displayed hyperactivity after activation, and the motion parameters and HYP were similar to those of the TL-BSA control. The PCP 200-μM treatment group displayed some impairments of hyperactivity after activation, such as low VCL and ALH as well as high STR, and the HYP was significantly lower when compared to the TL-BSA control group.
TABLE 2.
Computer-assisted semen analysis of sperm treated with ACH or PCP in glucose-containing media (data are presented as mean ± SEM; N = 3).
Mitochondrial Integrity and Membrane Potential
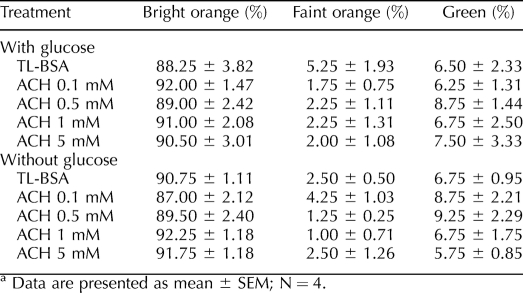
The MtoTracker Red accumulates and fluoresces in active sperm mitochondria after 30 min of incubation. Sperm treated with TL-BSA and 50 μM PCP in glucose-containing media showed similar mitochondrial labeling indicated by the brightly labeled midpieces (Fig. 3). The sperm in the ACH 0.5-mM treatment group consistently showed more intense mitochondrial labeling compared to those of the TL-BSA control group, suggesting that the mitochondria were more active. In contrast, sperm treated with 200 μM PCP showed no MitoTracker Red labeling (Fig. 3), indicating that the mitochondria were damaged and had lost membrane potential. To further investigate the mitochondrial membrane potential after the treatments, the sperm mitochondria were labeled with JC-1, and the percentage of sperm with active (bright orange), partial active (faint orange) and inactive (green) mitochondria was scored (Table 3). After 1 h of treatment, sperm treated with 200 μM PCP showed significantly lower numbers of sperm with active mitochondria (P < 0.05) and significantly higher numbers of sperm with inactive mitochondria (P < 0.05). After 30 min of chemical activation, the majority of sperm mitochondria (95%) in the PCP 200-μM treatment group were labeled inactive (green). No significant difference was observed when the percentage of different color-labeled sperm pre- and postactivation in each group was compared. Sperm treated with different levels of ACH in glucose or nonglucose media did not show significant difference in JC-1 staining, suggesting a lack of direct effect on mitochondria (Table 4).
FIG. 3.
Mitochondria integrity after 1 h of treatment in glucose-containing media. Sperm were stained with MitoTracker Red and mounted with Vectashield mounting medium with DAPI to examine mitochondria integrity. Sperm in the TL-BSA control and PCP 50-μM treatment groups showed strong and similar level of MitoTracker Red labeling in the midpieces, while sperm in the PCP 200-μM treatment group showed no MitoTracker Red labeling. In contrast, sperm in the ACH 0.5-mM treatment group showed intensive MitoTracker Red labeling, indicating that the mitochondria were very active.
TABLE 3.
Percentage of sperm mitochondria labeled with bright orange, faint orange, and green JC-1 after ACH or PCP treatment.a
TABLE 4.
Percentage of JC-1 labeled sperm treated with ACH in glucose or non-glucose media.a
Sperm Intracellular ATP Level
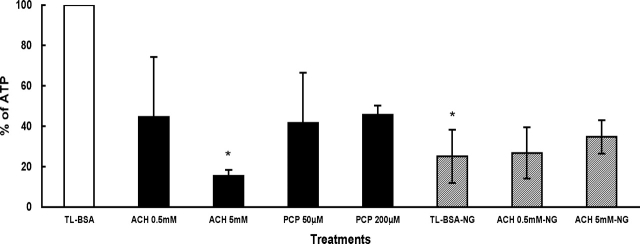
The sperm intracellular ATP level after ACH and PCP in the presence and absence of glucose is shown in Figure 4. The data are presented as percentages when compared to the TL-BSA control group (open bar). In glucose-containing media, sperm treated with ACH or PCP (closed bars) showed decreased intracellular ATP level compared to the TL-BSA control group (open bar). The most marked effect of ACH on ATP levels was seen after 5 mM ACH treatment where the change was found to be statistically significant (P < 0.05). In nonglucose media (patterned bars), the intracellular ATP level was initially much lower than in glucose-containing media but did not change with ACH treatments when compared to the TL-BSA control group. When comparing the sperm in TL-BSA with or without glucose, the sperm in nonglucose TL-BSA (TL-BSA-NG) showed a significant decrease (P < 0.05) of intracellular ATP level when compared to the sperm glucose-containing TL-BSA (open bar).
FIG. 4.
Intracellular ATP level after 1 h of treatment. The level of ATP is presented as percentage, and the TL-BSA with glucose is presented as 100% (open bar). The other treatments with glucose-containing media are shown in closed bars. Sperm treated in media without glucose (NG) are shown in patterned bars. Data are from three males and presented as % ± SEM. *, P < 0.05 when comparing mean differences with the TL-BSA control group.
Percentage of Sperm with Protein Tyrosine Phosphorylation
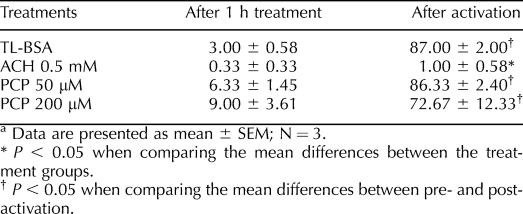
The results of the sperm tyrosine phosphorylation labeling experiment are shown in Table 5. After 1 h of treatment with ACH or PCP in glucose-containing media, low levels of sperm tyrosine phosphorylation in the principal piece of the flagellum were measured. After 30 min of chemical activation, all groups except for the ACH 0.5-mM treatment group showed a large and statistically significant increase in the percentage of sperm with tyrosine phosphorylation. Sperm treated with ACH 0.5 mM failed to demonstrate any change in protein tyrosine phosphorylation after treatment with dbcAMP.
TABLE 5.
Percentage of sperm with tyrosine phosphorylation after 1 h of treatment with ACH or PCP in glucose-containing media.a
Sperm-Zona Binding and Zona-Induced Acrosome Reaction
Sperm treated with ACH and PCP in glucose-containing media showed decreased numbers of sperm bound to the zona pellucida compared to the TL-BSA control group; however, the differences were not statistically significant (Table 6). The percentage of acrosome-reacted sperm induced by the zona pellucida in all treatment groups was similar to the TL-BSA control group.
TABLE 6.
Number of sperm bound to zona and percentage of acrosome reaction after 1 h of treatment with ACH or PCP in glucose-containing media.a
DISCUSSION
The ability of sperm to swim to the oocyte is crucial to achieve fertilization. The source of energy to drive flagellar movement of sperm has been thought to be from the mitochondria, as oxidative phosphorylation from mitochondrial respiration is more efficient than glycolysis for ATP production. However, there is debate about whether ATP produced by the mitochondria can be effectively delivered from the mitochondria supply the entire flagellum [7–9]. In order to address alternate possibilities, studies have focused on glycolysis as a possible energy source. In the present study, by using ACH and PCP, a glycolysis inhibitor and an oxidative phosphorylation inhibitor, we have demonstrated that glycolysis is the major energy source and more important than oxidative phosphorylation to support sperm motility, hyperactivation, and protein tyrosine phosphorylation in rhesus monkey sperm.
ACH has a structure similar to glycerol and is oxidized to 3-chloroactaldehyde by a NADP-dependent dehydrogenase that converts glycerol to glyceraldehyde [35]. 3-Chloroaceataldehyde inhibits GAPDH most likely because of its structural similarity to the GAPDH substrate, glyceraldehyde 3-phosphate, and consequently inhibits ATP production via glycolysis [36]. Sperm treated with ACH in vitro showed reduced fertilizing capacity in rats and mice [37]. Oral administration of ACH for 10 days resulted in reduced GAPDH activity in proximal and distal epididymal sperm in mice [38]. An acute study with ACH found inhibition of GAPDH activity not only in sperm but also in other tissues, although the inhibitory effect in sperm was greater than in other tissues [25]. Studies in vitro with mouse or rat sperm have also shown that without decreasing the percentage of motile sperm, 1 mM ACH decreased sperm motion parameters such as VCL, VSL, and LIN as well as glycolytic enzyme activities such as GAPDH and triosephosphate isomerase after 2 h of incubation [23, 39]. However, previous studies showed that the level of ATP was not decreased significantly in rhesus monkey sperm when treated with 0.5 mM ACH for 1 h in glucose-containing medium [40, 41]. In contrast with the later rodent studies [23, 39], an earlier study found that ACH did not decrease the ATP content in rat sperm [42]. Our results showed that the ATP level was lower in the 0.5-mM ACH treatment group (closed bar) when compared to the TL-BSA control group (open bar) while glucose was present; however, the decrease was not statistically significant, which is consistent with the earlier studies in rhesus monkey sperm [40, 41]. It is possible that active mitochondria seen to be intensely labeled with MitoTracker Red in the 0.5-mM ACH treatment group partially compensated for ACH-dependent loss of ATP (Fig. 3). An in vivo study in rhesus macaque also showed that ACH increased sperm oxygen consumption, which supports our finding in mitochondrial labeling intensity [43]. Although ATP was not significantly depleted at the 0.5-mM dose level, there was a marked effect on motility (Table 2), suggesting that mitochondrial ATP might not be delivered in sufficient quantity along the length of the flagellum to sustain normal sperm motility (Table 2) [7–9]. A earlier study had shown that a glycolytic product is required for whiplash motility during fertilization in mice [44]. We found that without affecting the percentage of motile sperm (Fig. 2), ACH impaired not only the whiplash motility (ALH, BCF) but also the velocity of motility (VAP, VSL, VCL) after 1 h of treatment in glucose-containing media (Table 2).
In the presence of glucose, ACH also inhibited hyperactivation of rhesus monkey sperm that is induced by the chemical activators dbcAMP and caffeine (Table 2). Studies have shown that ATP production through glycolysis is required for hyperactivated sperm motion in humans and mice [44–46]. Those studies support our findings that when glycolysis is inhibited, despite the presence of chemical activators, the ATP level in sperm maintained by highly active mitochondria is not adequate to support the high energy demand needed for hyperactivation.
Protein tyrosine phosphorylation is one of the characteristics that sperm acquire during capacitation and has been correlated to sperm hyperactivation in humans, nonhuman primates, and other species [47–49]. Our study showed that ACH also inhibits protein tyrosine phosphorylation after chemical activation (Table 5). Other investigators have demonstrated that ATP produced by glycolysis is required for protein tyrosine phosphorylation in mouse sperm [17], which is consistent with our finding that inhibiting glycolysis with ACH prevents protein tyrosine phosphorylation after chemical activation. Apparently, without displaying hyperactivity and tyrosine phosphorylation, the sperm can still bind to the zona pellucida and undergo zona-induced acrosome reaction (Table 6), which suggests that sperm-zona interaction and sperm hyperactivity have different energy requirements. Our previous study in cynomolgus macaques has shown that sperm-zona binding ability and zona-induced acrosome reaction are independent of sperm hyperactive motility; when treated with caffeine or dbcAMP only, increased numbers of sperm bound to the zona pellucida, and an increased percentage showed a zona-induced acrosome reaction yet without displaying hyperactive motility [50]. A study in human sperm has shown that protein tyrosine phosphorylation is correlated with sperm-zona binding but not with the zona-induced acrosome reaction [51]. In our study, the number of sperm bound to the zona pellucida was decreased but not statistically significant compared to the TL-BSA control, and the zona-induced acrosome reaction was not affected (Table 6). Previous studies in cynomolgus macaques and mice have shown that when coincubating sperm and oocytes in culture medium without glucose, the binding of sperm to the oocytes was retarded [20, 52]. With the support of previous findings [20, 44–46], our results suggest that glycolysis is not required for sperm-zona binding, zona-induced acrosome reaction, or capacitation but is essential for hyperactivated motility and protein tyrosine phosphorylation in rhesus monkey sperm.
PCP is an uncoupler of oxidative phosphorylation and inhibits respiratory enzymes to prevent ATP production in the mitochondria [53]. The PCP 50-μM treatment showed no effect on the motion parameters of sperm after 1 h of treatment and after activation (Table 2), and the mitochondria remained intact after treatment (Fig. 3 and Table 3). When treated with a higher level of PCP (200 μM), sperm mitochondrial activity was completely disrupted and displayed no MitoTracker Red labeling after 1 h incubation (Fig. 3); when labeled with JC-1, the majority of mitochondria (81%) showed inactive staining (green) (Table 3). Surprisingly, without active mitochondria, the sperm were still motile and showed no difference in the percentage of motile sperm compared to the TL-BSA treatment group (Fig. 2). Although the sperm motion parameters in the PCP 200-μM treatment group showed significant changes compared to the TL-BSA treatment group after 1 h of treatment, the changes were much smaller than those in the ACH treatment group (Table 2). A study has shown that when treated with another oxidative phosphorylation chemical, carbonyl cyanide m-chlorophenylhydrazone (CCCP), mouse sperm motility was not impaired [54]. Our results and these other studies suggest that the ATP produced via glycolysis alone is sufficient to support sperm motility.
After chemical activation, sperm in both PCP treatment groups displayed the characteristic motion of hyperactivity, such as increased VCL and ALH and decreased BCF, STR, and LIN. Sperm treated with 50 μM PCP showed no difference in sperm motion parameters when compared to the TL-BSA control group. Sperm treated with 200 μM PCP also showed some degree of hyperactivity (although lower HYP) after chemical activation despite the fact that the sperm motility was affected by 1 h of PCP treatment; only VCL, ALH, and STR showed significant differences compared to the TL-BSA control group after activation (Table 2). These results suggest that the sperm mitochondria may play a secondary role in maintaining normal sperm motility and hyperactive motility because PCP impairment of sperm motility can be rescued after chemical activation. Although sperm treated with 200 μM PCP showed some impairments of hyperactivity, the ability of sperm to bind to the zona pellucida was not significantly affected, and the sperm could undergo zona-induced acrosome reaction (Table 6). In addition, sperm showed normal protein tyrosine phosphorylation after chemical activation (Table 5), which is consistent with the finding in mouse sperm that when treated with oxidative phosphorylation uncouplers and electron transfer inhibitors, protein tyrosine phosphorylation was not inhibited [55]. Our findings provide further support that glycolysis but not oxidative phosphorylation is essential for protein tyrosine phosphorylation in rhesus monkey sperm.
There appears to be a species difference in sensitivity of sperm mitochondria to PCP. In rats, 0.1 μM of PCP significantly disrupted mitochondrial membrane potential [26]. In our study, the mitochondria were affected only when treated with 200 μM PCP (Fig. 3), indicating that monkey sperm are more tolerant to PCP. The EC50 value to affect bull sperm motility, velocity, and viability are 43, 27, and 360 μM, respectively; however, the ATP level was not affected when treated with 100 μM PCP [27]. Our study showed that the percentage of motile sperm was not changed even with PCP 200-μM treatment. The velocity of sperm motion was decreased in the 200-μM PCP treatment group (Table 2), which is a much higher dose level than that producing an effect in bull sperm. These species sensitivity differences may be a confounding factor in previous studies that have evaluated whether oxidative phosphorylation or glycolysis is more important for energy production to support sperm function.
It is hypothesized that incubating sperm with ACH in glucose-containing medium would increase the concentration of glycolytic intermediates that bind most of the phosphate in the sperm, consequently reducing the availability of phosphate for oxidative phosphorylation and decreasing of sperm motility, and this is likely to increase rather than decrease mitochondrial membrane potential by reducing the availability of free phosphate for ATP synthesis [18]. Sperm treated with ACH showed intensive MitoTracker Red staining, suggesting that the mitochondrial membrane potential was increased, perhaps because of the lack of free phosphate for ATP synthesis (Fig. 3); however, some studies propose that an increase of membrane potential might indicate a higher rate of mitochondrial ATP synthesis, which may also suggest a more active mitochondria perhaps to compensate for diminished ATP production [56, 57]. Previous studies in rhesus monkey sperm have shown that ATP level was maintained and oxygen consumption was increased when treated with ACH [40, 43], supporting the possibility that insufficient delivery of ATP diminished sperm motion after ACH treatment despite the active mitochondria. In addition, our PCP data strongly suggest that without functional mitochondria, monkey sperm still displayed hyperactivated motility.
We conclude that glycolytic pathways play an important role in supplying ATP to support sperm motility in rhesus monkey sperm. While ATP from mitochondrial sources does contribute to sperm motility, our data indicate that the mitochondria appear to be less important for hyperactivated motility. Despite the absence of hyperactive motility after chemical activation, sperm treated with either ACH or PCP can bind to the zona pellucida and undergo zona-induced acrosome reaction, indicating that the ATP from either glycolysis or oxidative phosphorylation alone is sufficient to provide the energy required for sperm to capacitate and bind to the zona pellucida then acrosome react, at least in the in vitro conditions. Sperm mitochondria have been used to assess the viability of sperm for decades. With the inconsistency of mitochondrial activity and sperm motility in this study and others [54], mitochondrial integrity is not necessarily the best primary index of sperm viability.
Acknowledgments
The authors thank Dr. Julie Baumber and Megan McCarthy for CASA assistance.
Footnotes
1Supported by Philip Morris External Research Program, NIH grant RR00169, and by R01 RR016581.
REFERENCES
- Halangk W, Troger U, Bohnensack R.Quantification of aerobic energy turnover in epididymal bull spermatozoa. Biochim Biophys Acta 1990; 1015: 243–247. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Lapena AC, Diez-Sanchez C, Perez-Martos A, Montoya J, Alvarez E, Diaz M, Urrieis A, Montoro L, Lopez-Perez MJ, Enriquez JA.Human mtDNA haplogroups associated with high or reduced spermatozoa motility. Am J Hum Genet 2000; 67: 682–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzosiak J, Molan P, Vishwanath R.Measurements of bovine sperm velocities under true anaerobic and aerobic conditions. Anim Reprod Sci 1999; 55: 163–173. [DOI] [PubMed] [Google Scholar]
- Halangk W, Bohnensack R.Quantification of sperm motility by a turbidimetric assay—correlation to cellular respiration. Biomed Biochim Acta 1986; 45: 331–341. [PubMed] [Google Scholar]
- Halangk W, Bohnensack R, Frank K, Kunz W.Effect of various substrates on mitochondrial and cellular-energy state of intact spermatozoa. Biomed Biochim Acta 1985; 44: 411–420. [PubMed] [Google Scholar]
- Ford WCL, Harrison A.The role of oxidative-phosphorylation in the generation of ATP in human-spermatozoa. J Reprod Fertil 1981; 63: 271–278. [DOI] [PubMed] [Google Scholar]
- Du J, Tao J, Kleinhans FW, Mazur P, Critser JK.Water volume and osmotic behaviour of mouse spermatozoa determined by electron paramagnetic resonance. J Reprod Fertil 1994; 101: 37–42. [DOI] [PubMed] [Google Scholar]
- Nevo AC, Rikmenspoel R.Diffusion of ATP in sperm flagella. J Theor Biol 1970; 26: 11–18. [DOI] [PubMed] [Google Scholar]
- Adam DE, Wei J.Mass-transport of ATP within motile sperm. J Theor Biol 1975; 49: 125–145. [DOI] [PubMed] [Google Scholar]
- Storey BT, Kayne FJ.Energy metabolism of spermatozoa. V. The Embden-Myerhof pathway of glycolysis: activities of pathway enzymes in hypotonically treated rabbit epididymal spermatozoa. Fertil Steril 1975; 26: 1257–1265. [PubMed] [Google Scholar]
- Travis AJ, Foster JA, Rosenbaum NA, Visconti PE, Gerton GL, Kopf GS, Moss SB.Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa. Mol Biol Cell 1998; 9: 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MP, Geelan A, Leitch V, Goldberg E.Cloning sequencing and characterization of LDH-C-4 from a fox testis cDNA library. Mol Reprod Dev 1996; 44: 452–459. [DOI] [PubMed] [Google Scholar]
- Kim YH, Haidl G, Schaefer M, Egner U, Mandal A, Herr JC.Compartmentalization of a unique ADP/ATP carrier protein SFEC (Sperm Flagellar Energy Carrier, AAC4) with glycolytic enzymes in the fibrous sheath of the human sperm flagellar principal piece. Dev Biol 2007; 302: 463–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin S, Chiang K, Bassilian S, Lee WNP, Boros LG, Fernandez-Novell JM, Centelles JJ, Medrano A, Rodriguez-Gil JE, Cascante M.Metabolic strategy of boar spermatozoa revealed by a metabolomic characterization. FEBS Lett 2003; 554: 342–346. [DOI] [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA.Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A 2004; 101: 16501–16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urner F, Leppens-Luisier G, Sakkas D.Protein tyrosine phosphorylation in sperm during gamete interaction in the mouse: the influence of glucose. Biol Reprod 2001; 64: 1350–1357. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, Storey BT, Kopf GS, Moss SB.Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem 2001; 276: 7630–7636. [DOI] [PubMed] [Google Scholar]
- Ford WCL.Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update 2006; 12: 269–274. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Takahashi T, Iguchi N, Kitamura K, Miyagawa Y, Tsujimura A, Matsumiya K, Okuyama A, Nishimune Y.Ketone bodies could support the motility but not the acrosome reaction of mouse sperm. Int J Androl 2004; 27: 172–177. [DOI] [PubMed] [Google Scholar]
- Redkar AA, Olds-Clarke PJ.An improved mouse sperm-oocyte plasmalemma binding assay: studies on characteristics of sperm binding in medium with or without glucose. J Androl 1999; 20: 500–508. [PubMed] [Google Scholar]
- Jacobs JM, Ford WC.The neurotoxicity and antifertility properties of 6-chloro-6-deoxyglucose in the mouse. Neurotoxicology 1981; 2: 405–417. [PubMed] [Google Scholar]
- Ford WCL, Waites GMH.Activities of various 6-chloro-6-deoxysugars and (S)alpha-chlorohydrin in producing spermatoceles in rats and paralysis in mice and in inhibiting glucose-metabolism in bull spermatozoa in vitro. J Reprod Fertil 1982; 65: 177–183. [DOI] [PubMed] [Google Scholar]
- Bone W, Jones AR, Morin C, Nieschlag E, Cooper TG.Susceptibility of glycolytic enzyme activity and motility of spermatozoa from rat, mouse, and human to inhibition by proven and putative chlorinated antifertility compounds in vitro. J Androl 2001; 22: 464–470. [PubMed] [Google Scholar]
- Kirton KT, Ericsson RJ, Ray JA, Forbes AD.Male antifertility compounds—efficacy of U-5897 in primates (Macaca-Mulatta). J Reprod Fertil 1970; 21: 275–278. [DOI] [PubMed] [Google Scholar]
- Jelks KB, Miller MG.Alpha-chlorohydrin inhibits glyceraldehyde-3-phosphate dehydrogenase in multiple organs as well as in sperm. Toxicol Sci 2001; 62: 115–123. [DOI] [PubMed] [Google Scholar]
- Gravance CG, Garner DL, Miller MG, Berger T.Flow cytometric assessment of changes in rat sperm mitochondrial function after treatment with pentachlorophenol. Toxicol In Vitro 2003; 17: 253–257. [DOI] [PubMed] [Google Scholar]
- Seibert H, Kolossa M, Wassermann O.Bovine spermatozoa as an in vitro model for studies on the cytotoxicity of chemicals: effects of chlorophenols. Cell Biol Toxicol 1989; 5: 315–330. [DOI] [PubMed] [Google Scholar]
- Sarason RL, VandeVoort CA, Mader DR, Overstreet JW.The use of nonmetal electrodes in electroejaculation of restrained but unanesthetized macaques. J Med Primatol 1991; 20: 122–125. [PubMed] [Google Scholar]
- Wolf DP, VandeVoort CA, Meyer-Haas GR, Zelinski-Wooten MB, Hess DL, Baughman WL, Stouffer RL.In vitro fertilization and embryo transfer in the rhesus monkey. Biol Reprod 1989; 41: 335–346. [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Tollner TL, Overstreet JW.Sperm-zona pellucida interaction in cynomolgus and rhesus macaques. J Androl 1992; 13: 428–432. [PubMed] [Google Scholar]
- Hung PH, Baumber J, Meyers SA, VandeVoort CA.Effects of environmental tobacco smoke in vitro on rhesus monkey sperm function. Reprod Toxicol 2007; 23: 499–506. [DOI] [PubMed] [Google Scholar]
- Baumber J, Meyers SA.Hyperactivated motility in rhesus macaque (Macaca mulatta) spermatozoa. J Androl 2006; 27: 459–468. [DOI] [PubMed] [Google Scholar]
- Jelks K, Berger T, Horner C, Miller MG.[alpha]-Chlorohydrin induced changes in sperm fertilizing ability in the rat: association with diminished sperm ATP levels and motility. Reprod Toxicol 2000; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Yudin AI, Overstreet JW.Interaction of acrosome-reacted macaque sperm with the macaque zona pellucida. Biol Reprod 1997; 56: 1307–1316. [DOI] [PubMed] [Google Scholar]
- Stevenson D, Jones AR.Production of (S)-3-chlorolactaldehyde from (S)-alpha-chlorohydrin by boar spermatozoa and the inhibition of glyceraldehyde-3-phosphate dehydrogenase in vitro. J Reprod Fertil 1985; 74: 157–165. [DOI] [PubMed] [Google Scholar]
- Jones AR.Chemical interference with sperm metabolic pathways. J Reprod Fertil Suppl 1998; 53: 227–234. [PubMed] [Google Scholar]
- Tsunoda Y, Chang MC.Fertilizing ability in vivo and in vitro of spermatozoa of rats and mice treated with alpha-chlorohydrin. J Reprod Fertil 1976; 46: 401–406. [DOI] [PubMed] [Google Scholar]
- Ford WCL, Harrison A.The activity of glyceraldehyde-3-phosphate dehydrogenase in spermatozoa from different regions of the epididymis in laboratory rodents treated with alpha-chlorohydrin or 6-chloro-deoxyglucose. J Reprod Fertil 1983; 69: 147–156. [DOI] [PubMed] [Google Scholar]
- Bone W, Cooper TG.In vitro inhibition of rat cauda epididymal sperm glycolytic enzymes by ornidazole, alpha-chlorohydrin and 1-chloro-3-hydroxypropanone. Int J Androl 2000; 23: 284–293. [DOI] [PubMed] [Google Scholar]
- Ford WCL, Harrison A, Takkar GL, Waites GMH.Inhibition of glucose catabolism in rat, hamster, rhesus-monkey and human-spermatozoa by alpha-chlorohydrin. Int J Androl 1979; 2: 275–288. [Google Scholar]
- Ford WCL, Harrison A.Effect of alpha-chlorohydrin on glucose-metabolism by spermatozoa from the cauda epididymidis of the rhesus-monkey (Macaca-Mulatta). J Reprod Fertil 1980; 60: 59–64. [DOI] [PubMed] [Google Scholar]
- Chulavatnatol M, Hasibuan I, Yindepit S, Eksittikul T.Lack of effect of alpha-chlorohydrin on the ATP content of rat, mouse and human spermatozoa. J Reprod Fertil 1977; 50: 137–139. [DOI] [PubMed] [Google Scholar]
- Setty BS, Kar AB, Roy SK, Chowdhury SR.Studies with sub-toxic doses of [alpha]-chlorohydrin in the male monkey. Contraception 1970; 1: 279–289. [Google Scholar]
- Fraser LR, Quinn PJ.A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whiplash motility required for fertilization in the mouse. J Reprod Fertil 1981; 61: 25–35. [DOI] [PubMed] [Google Scholar]
- Hoshi K, Tsukikawa S, Sato A.Importance of Ca2+, K+ and glucose in the medium for sperm penetration through the human zona pellucida. Tohoku J Exp Med 1991; 165: 99–104. [DOI] [PubMed] [Google Scholar]
- Urner F, Sakkas D.Glucose participates in sperm-oocyte fusion in the mouse. Biol Reprod 1996; 55: 917–922. [DOI] [PubMed] [Google Scholar]
- Nassar A, Mahony M, Morshedi M, Lin MH, Srisombut C, Oehninger S.Modulation of sperm tail protein tyrosine phosphorylation by pentoxifylline and its correlation with hyperactivated motility. Fertil Steril 1999; 71: 919–923. [DOI] [PubMed] [Google Scholar]
- Naz R, Rajesh P.Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod Biol Endocrinol 2004; 2: 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony MC, Gwathmey T.Protein tyrosine phosphorylation during hyperactivated motility of cynomolgus monkey (Macaca fascicularis) spermatozoa. Biol Reprod 1999; 60: 1239–1243. [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Tollner TL, Overstreet JW.Separate effects of caffeine and dbcAMP on macaque sperm motility and interaction with the zona pellucida. Mol Reprod Dev 1994; 37: 299–304. [DOI] [PubMed] [Google Scholar]
- Liu DY, Clarke GN, Baker HWG.Tyrosine phosphorylation on capacitated human sperm tail detected by immunofluorescence correlates strongly with sperm-zona pellucida (ZP) binding but not with the ZP-induced acrosome reaction. Hum Reprod 2006; 21: 1002–1008. [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Overstreet JW.Effects of glucose and other energy substrates on the hyperactivated motility of macaque sperm and the zona pellucida-induced acrosome reaction. J Androl 1995; 16: 327–333. [PubMed] [Google Scholar]
- Weinbach EC, Garbus J.Interaction of uncoupling phenols with mitochondria and with mitochondrial protein. J Biol Chem 1965; 240: 1811–1819. [PubMed] [Google Scholar]
- Mukai C, Okuno M.Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod 2004; 71: 540–547. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Tutuncu L, Jorgez CJ, Ord TS, Jones BH, Kopf GS, Williams CJ.Requirements for glucose beyond sperm capacitation during in vitro fertilization in the mouse. Biol Reprod 2004; 71: 139–145. [DOI] [PubMed] [Google Scholar]
- Chen LB.Mitochondrial membrane potential in living cells. Annu Rev Cell Biol 1988; 4: 155–181. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Walsh ML, Bockus BJ, Chen LB.Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol 1981; 88: 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]