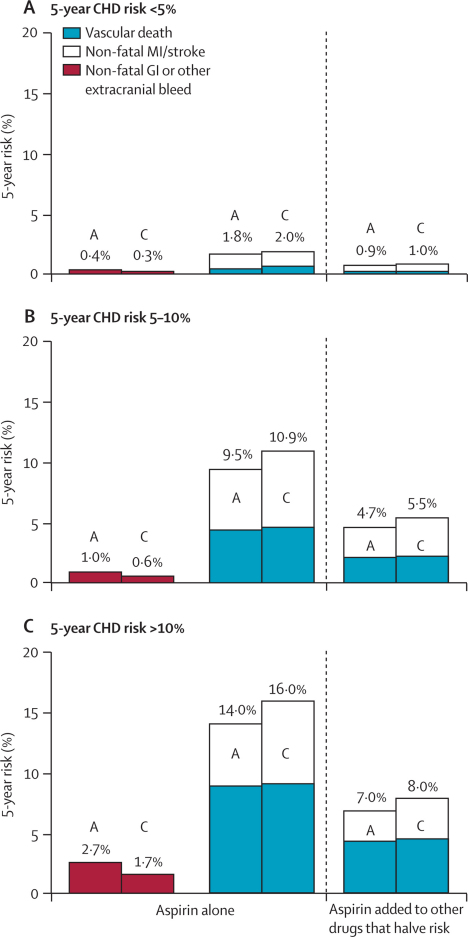
Figure 7.
Predicted 5-year absolute effects of allocation to aspirin in the primary prevention trials in different categories of 5-year risk (if untreated) of coronary heart disease (CHD) (ignoring non-vascular mortality)
Three outcomes were analysed in aspirin (A) and control (C) groups: non-fatal gastrointestinal (GI) (or other non-cerebral) bleeds when aspirin is given alone; non-fatal vascular events when aspirin is given alone and when aspirin is added to other drugs that halve risk; and vascular mortality (including any fatal bleeds) when aspirin is given alone and when aspirin is added to other drugs that halve risk. For every outcome, the overall risk ratio, irrespective of risk of coronary heart disease, was combined with the absolute yearly risk among the controls in three categories of predicted 5-year risk of a major coronary event (<5%, 5–10%, >10%). Absolute effects are estimated both directly from the data (middle column) and in the hypothetical situation in which risk is halved by statins and other primary prevention measures (right-hand column).