Table 1.
Salt effect
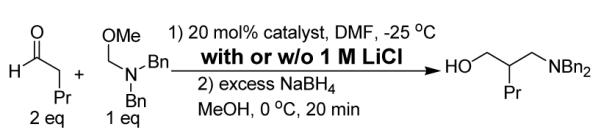 | |||||
|---|---|---|---|---|---|
| entrya | catalyst | salt | time | eeb | favored enantiomerc |
| 1 | L-proline | -- | 24 h | 49 | S |
| 2 | L-proline | LiCl | 24 h | <5 | -- |
| 3 | C-HOAc | -- | 2 h | 67 | R |
| 4 | C-HOAc | LiCl | 2 h | 80 | R |
Yield of all reactions > 80% as measured by 1H NMR of the crude reaction mixture before reduction; the reduction is quantitative.
Determined by chiral phase HPLC.
See supporting information for stereochemistry determination.
