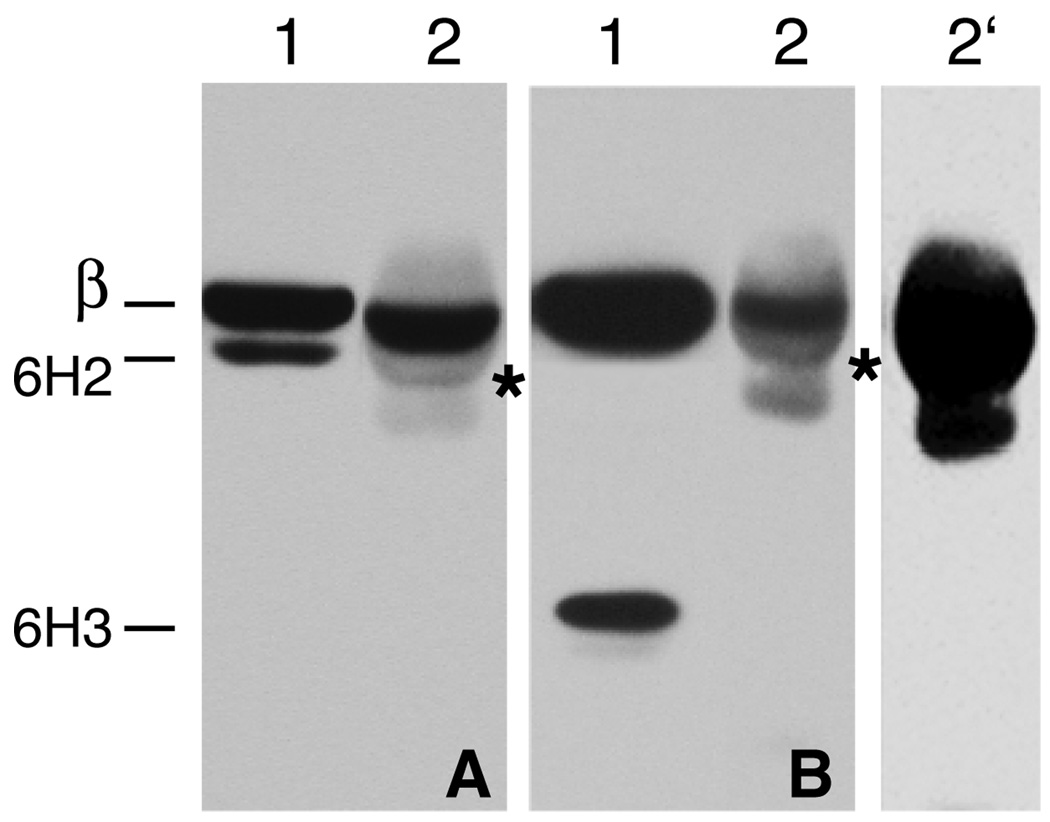
Fig. 4.
Immunoprecipitation of tubulin heterodimers. Cell extracts from revertants 6H2 (panel A) and 6H3 (panel B) were incubated with a rabbit antibody to α-tubulin coupled to protein G Sepharose beads. The immunoprecipitates were analyzed by Western blotting with a mouse antibody specific for the N-terminus of β-tubulin. Lane 1 in each of the two panels represents the unfractionated cell extract and lane 2 represents the immunoprecipitate. Lane 2’ represents an overexposure of lane 2 from panel B to demonstrate that no 6H3 mutant tubulin is found in the immunoprecipitate. The light fuzzy bands and the weak sharp bands with a migration similar to 6H2 β-tubulin (asterisks) present in the immunoprecipitates are due to weak cross reactivity of the goat antimouse IgG secondary antibody with the rabbit IgG heavy chains. The migration of endogenous wild-type β-tubulin (β), 6H2 mutant β-tubulin (6H2) and mutant 6H3 β-tubulin (6H3) is indicated.