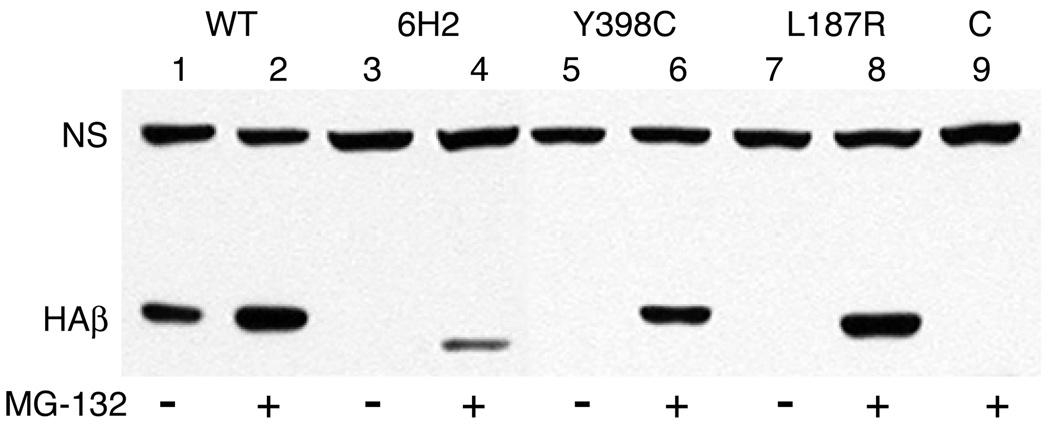
Fig. 7.
Inhibition of HAβ1-tubulin degradation. CHO cells were stably transfected with wild-type HAβ1-tubulin cDNA (WT, lanes 1 and 2) or with the same cDNA encoding a 12 amino acid deletion (6H2, lanes 3 and 4), a Y398C mutation (lanes 5 and 6), or an L187R mutation (lanes 7 and 8). The transfected cell lines were then treated with (+) or without (−) the proteasomal inhibitor MG132 for 8 h and lysed in SDS. Proteins were analyzed by Western blotting with a mouse antibody to the HA tag. Bands representing transfected HAβ1-tubulin (HAβ) and a nonspecific endogenous CHO protein that cross reacts with the antibody (NS) and acts as a loading control are labeled. “C” (lane 9) represents a control lysate from non-transfected cells that were treated with MG132.