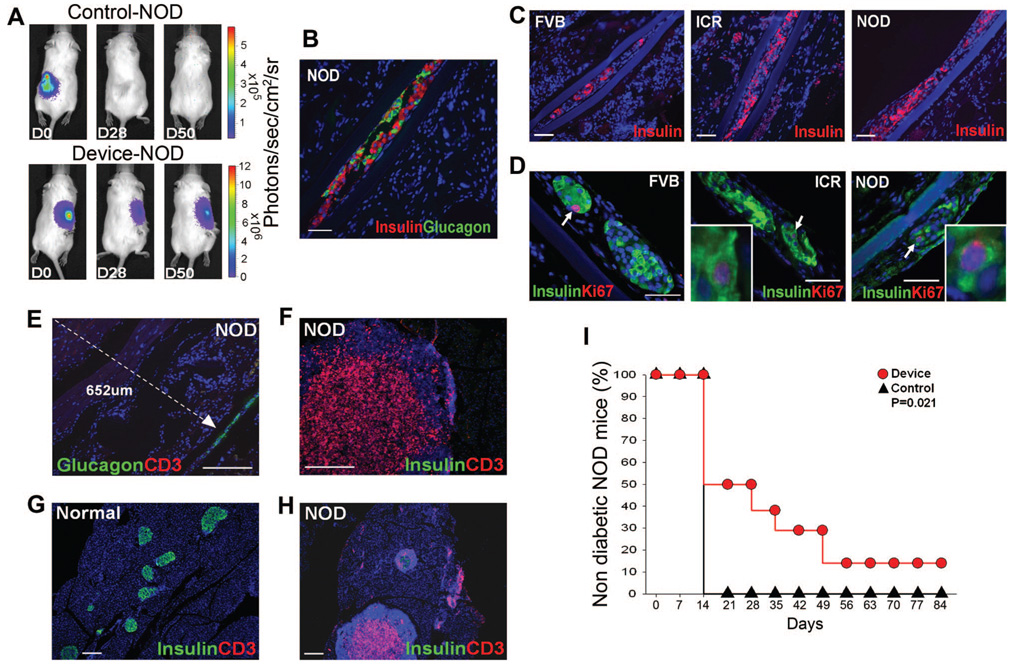
FIGURE 5.
Encapsulated islets survive and alleviate diabetes in NOD mice. (A) BLI of representative NOD mice transplanted with FVBluc islets, unencapsulated (Control, n = 7) or encapsulated (Device, n = 8). Animals were imaged immediately after transplantation (D0) and subsequently. (B) Insulin (red) and glucagon (green) immunostaining of representative device retrieved from NOD mouse after 50 days of transplantation when blood glucose levels were above 300 mg/dL, scale bar: 50 µm. (C) FVBluc islet-filled devices retrieved from wtFVB, islet cell resources, and NOD recipient mice and immunostained for insulin (red) reveal similar extent of β-cell survival in all strains, scale bar: 50 µm and (D) contain similar numbers of replicating β-cells (white arrows) as illustrated by immunostaining for insulin (green) and Ki67 (red), scale bar: 50 µm. Inset is higher magnification. (E) Immunostaining for glucagon (green) and T-cell marker CD3 (red) in device and surrounding tissue explanted from NOD mouse. Note that no T cells can be identified by anti-CD3 antibody (red) within 652 µm (white dotted line) of device (white arrowhead) (n = 10), scale bar: 200 µm. (F, H) Immunostaining for insulin (green) and T-cell marker CD3 (red) in pancreas from the same NOD mouse at (F) high and (H) low power, revealing massive CD3-positive infiltrates in pancreas, scale bar: 200 µm. (G) Normal pancreas, scale bar: 200 µm. (I) NOD mice transplanted with encapsulated islets (Device, red circle, n = 8) and untransplanted NOD mice (Control, black triangle, n = 7) received a single treatment of low-dose streptozotocin at day 0 to accelerate autoimmune diabetes (35, 36), P = 0.021. Mice with encapsulated islets exhibited delayed onset of diabetes compared with control animals. Nondiabetic blood glucose level is less than 300 mg/dL. Blue nuclear counterstaining B to H is DAPI.