Figure 1.
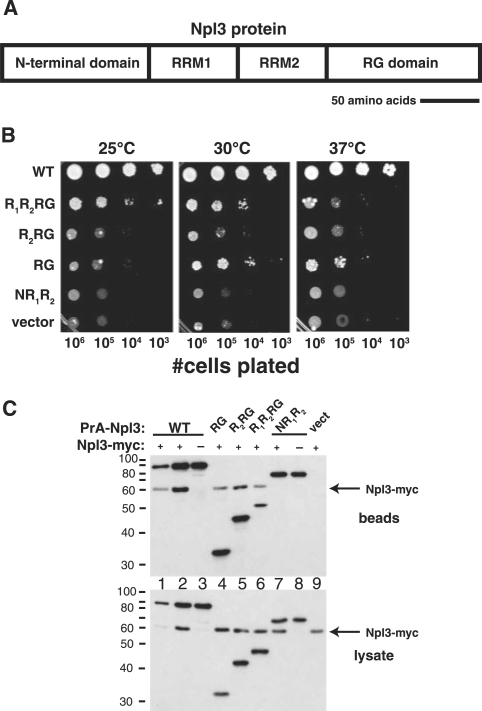
Role of the RG-rich domain in Npl3 self-association and function. (A) Schematic diagram of Npl3 protein primary structure including two RRMs and the arginine–glycine (RG)-rich C-terminal domain (total length = 414 amino acids, scale bar = 50 amino acids). (B) The RG domain alone can support partial growth of cells lacking Npl3. PSY814 (npl3Δ) bearing the plasmids described in (C) below was grown to mid-log-phase, plated on medium containing 5-FOA and lacking leucine, and grown for 4 days at 25°C, 3 days at 30°C or 3 days at 37°C. (C) The RG-rich domain is required for Npl3 self-association. YAM533 (hmt1ΔNPL3-myc) was transformed with plasmids expressing Protein A (PrA) fused to: full-length Npl3p (WT; lanes 1 and 2), the RG domain of Npl3 (RG; lane 4), the second RRM and the RG domain (R2RG; lane 5), both RRMs and the RG domain (R1R2RG; lane 6), or Npl3 lacking the RG domain (NR1R2; lane 7). Mid-log-phase cells were lysed, the PrA fusion protein isolated with IgG-sepharose, and co-precipitated Npl3-myc was detected (beads) by immunoblotting with a polyclonal anti-myc antibody (which also recognizes the PrA fusion protein). Controls included cells lacking Npl3-myc (lanes 3 and 8) and cells expressing only PrA (vect; lane 9). Expression of PrA fusion proteins and Npl3-myc was verified by anti-myc immunoblot analysis of the whole cell lysates used for purification (lysates).