Figure 2.
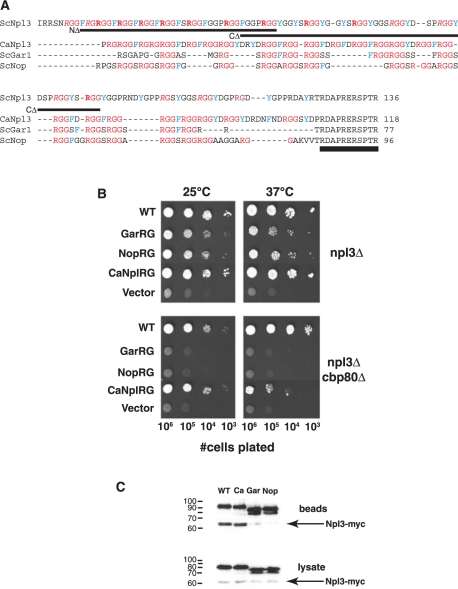
Specificity of the RG-rich domain in Npl3 function. (A) Comparison of RG domains of four yeast RNA-binding proteins. C-terminal sequences of chimeric Npl3 proteins containing RG domains from the C. albicans Npl3 ortholog and S. cerevisiae nucleolar proteins Gar1 and Nop 1 were aligned with ClustalW. Lines indicate the regions removed from the ScNpl3 RG domain in the N-terminal (NΔ) and C-terminal (CΔ) deletion mutants used in Figure 3. Note the presence of the C-terminal 11 amino acids of S. cerevisiae Npl3 on each chimeric protein (double underline). In ScNpl3, arginines that were previously found to be exclusively dimethylated are noted in bold and arginines with variable levels of methylation are italicized (16). (B) Heterologous RG domains can partially support growth of cells lacking Npl3. PSY814 (npl3Δ) and YAM505 (npl3Δcbp80Δ) were transformed with plasmids expressing chimeric Npl3 proteins containing the RG regions described in (A). Cells were tested for chimera function as described for Figure 1, with growth for 3 days (npl3Δ, 37°C) or 4 days (npl3Δ, 25°C; npl3Δcbp80Δ, 25°C, 37°C). (C) RG-domain specificity in Npl3 self-association. Plasmids in (B) were transformed into YAM533 and the ability of Npl3-myc to co-precipitate with PrA-tagged chimeric proteins was tested as in Figure 1.