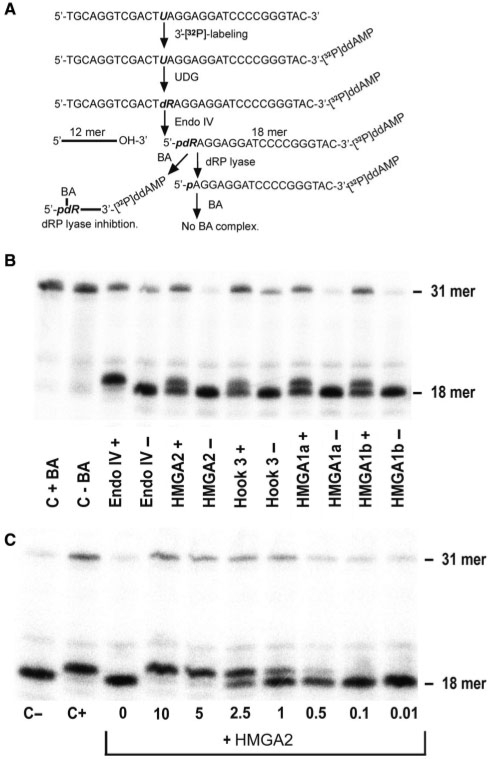
Figure 4.
Characterization of the dRP lyase activity. (A) Strategy for the creation of the dRP lyase substrate and expected cleavage products. (B) dRP lyase activity assay. The electrophoretic mobility of the 18-mer substrate containing the dRP moiety is retarted due to BA-adduct formation. The observed shift and visible double band verified that the HMGA proteins and hook 3 cleaved the substrate. (C) Quantitative in vitro analysis of BA as an inhibitor of the HMGA2 lyase activity. BA was first added to the reaction for 5 min, followed by HMGA incubation for 15 min.