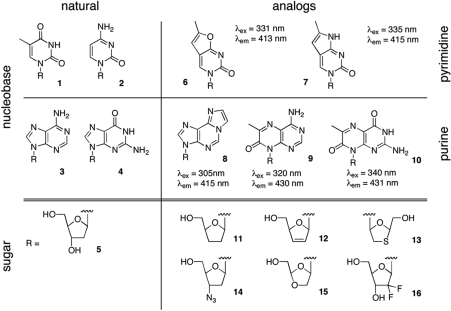
Figure 1.
Overview of modular nucleoside design. In many nucleoside analogs, a natural pyrimidine (1,2) or purine (3,4) moiety is docked to a sugar analog (11–16) instead of the native 2′-deoxyribosyl portion (5). For the creation of fluorescent NAs, an extension of the heterocycles, as exemplified in furano and pyrrolo-pyrimidines (6,7), as well as etheno-adenine (8) and pterines (9,10), red-shifts the excitation/emission spectrum of the nucleobase and increases the quantum yield. When coupled to sugar analogs (11–16), these fluorescent versions of the NA become useful molecular probes for studying their cellular uptake and metabolism (25).