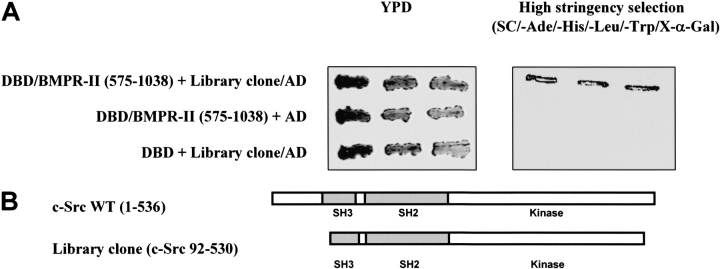
Figure 1.
Isolation of protein interacting with C-terminal domain of BMPR-II. (A) Growth of yeast transformants on YPD and high-stringency selection plates. One colony of each of the indicated yeast transformants was resuspended in 0.5 ml of water and streaked with three repeats onto rich yeast peptone dextrose (YPD) medium (left panel) or high-stringency selection plates containing X-α-Gal (right panel). Yeast cells were transformed with the following plasmids: (1) the C-terminal domain of BMPR-II (pGBKT7-DBD-BMPR-II [575–1038]) and the isolated positive library clone (pGADT7-library clone-AD) from the yeast two-hybrid screening; (2) C-terminal domain of BMPR-II and pGADT7 empty vector; and (3) pGBKT7-DBD empty vector and the isolated library clone. (B) Schematic representation of the library clone isolated from the yeast two-hybrid screening. The isolated library clone was identified by direct sequencing and consisted of partial sequence of the full-length c-Src tyrosine kinase. The numbers of the amino acid residues were indicated. The isolated library clone consisted of the Src Homology (SH) domains 2 and 3, and the catalytic tyrosine kinase.