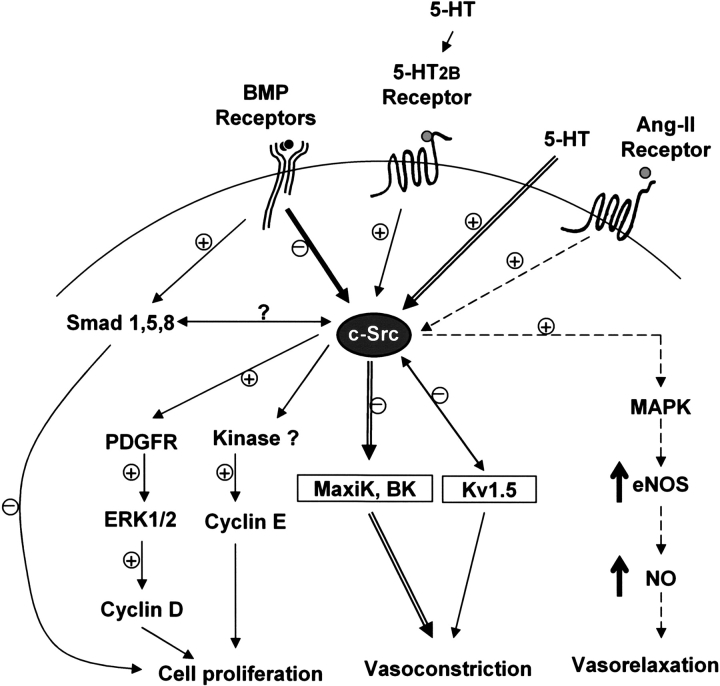
Figure 8.
Schematic representation of c-Src tyrosine kinase as a potential hub for signaling pathways involved in PAH. The interaction between BMPR-II and c-Src tyrosine kinase may inhibit c-Src tyrosine kinase activity in the presence of BMP ligand by reducing its phosphorylation at tyrosine-418 residue. The inhibition of c-Src activity by BMP signaling may inhibit downstream cell cycle regulators such as cyclins D and E and subsequently prevent smooth muscle cell proliferation. Moreover, BMP ligand stimulation may also inhibit cell proliferation through the Smad pathway. In contrast, activation of c-Src tyrosine kinase by 5-HT2B receptor causes the induction of cell cycle regulators (cyclins D and E) via ERK/MAPK pathway and subsequently triggers cell proliferation. In addition, activation of c-Src tyrosine kinase by serotonin inhibits voltage-dependent and Ca2+-activated K+ channel (MaxiK, BK), leading to vasoconstriction. Protein–protein interaction between c-Src tyrosine kinase and voltage-gated K+ channel (Kv1.5) suppresses outward potassium current and ultimately causes vasoconstriction. In contrast, activation of c-Src tyrosine kinase by angiotensin-II (Ang-II) receptor leads to increased eNOS gene expression and activities via MAPK pathway and subsequently causes vasorelaxation. The “+” and “−” signs indicated possible activation and inhibition, respectively, while “?” indicated potential cross-talk of the smad and c-Src signaling pathway.