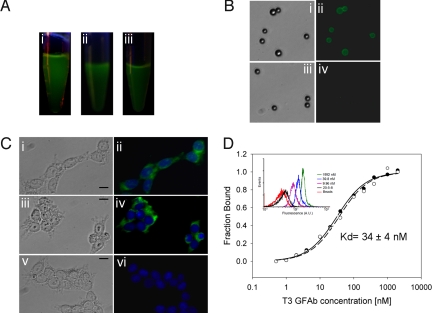
Fig. 2.
Properties of soluble, purified GFAbs. (A) Samples of purified protein illuminated with a hand-held UV lamp indicating the presence of fluorescent protein: i) GFPM, ii) 20–5-8, and iii) G6 GFAb. (B) Streptavidin-coated polystyrene beads were labeled with streptavidin-PE binding 1.3 GFAb (i and ii) or its parent scaffold 20–5-8 (iii and iv) and imaged by fluorescence microscopy. (C) Permeablized HEK-293 cells were incubated with: (i and ii) GAPDH binding G6 GFAb, (iii and iv) an anti-GAPDH antibody followed by fluorescent Alexa488 secondary antibody, and (v and vi) the parent scaffold 20–5-8, and cells were monitored for green fluorescence (GFAb-derived: i, ii, v, and vi or Alexa488-derived: iii and iv). DAPI was used to stain the nucleus (blue). (Scale bar, 20 μm.) (D) Affinity titration for secreted T3 GFAb on TrkB-loaded beads. Binding-dependent GFAb fluorescence was monitored by flow cytometry as a function of T3 concentration to generate a binding curve and associated KD. Representative flow cytometry histograms for various T3 dilutions are presented in the Inset. Also shown are no GFAb, bead-only controls, and the negligible binding of parent scaffold 20–5-8 to TrkB-loaded beads. Beads loaded with irrelevant antigen and labeled with T3 also exhibited negligible binding mirroring that shown for 20–5-8. Data from duplicate samples are depicted as open and filled circles along with their associated equilibrium binding model fits.