Figure 4.
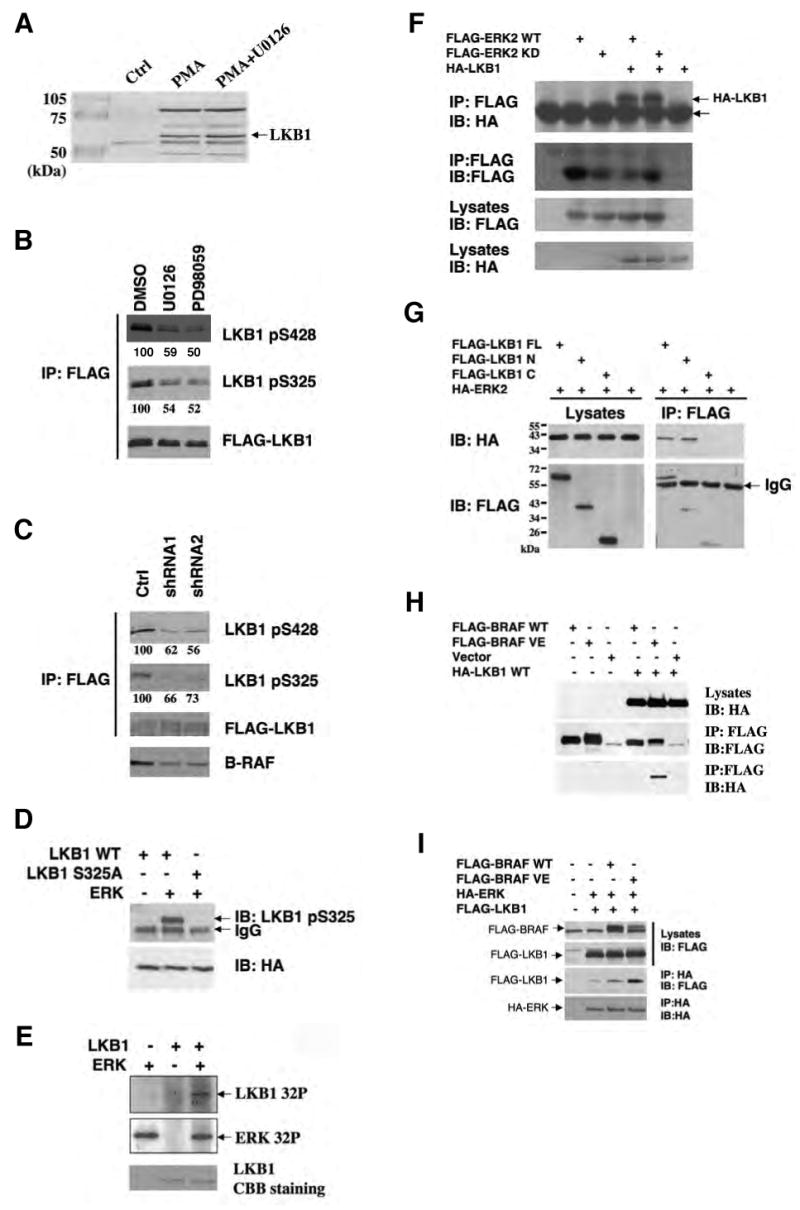
Phosphorylation of Ser325 and Ser428 of LKB1 by two downstream kinases of B-RAF, ERK and p90Rsk, respectively.
(A) Identification of phosophorylated LKB1 peptides containing Ser325 and Ser428 by LC-MS/MS analysis. HEK293 cells transfected with FLAG-LKB1 were serum-starved and pretreated with or without 20 μM of U0126 for 2 hr before the addition of 200 nM PMA for 20 min. FLAG-LKB1 proteins were immunoprecipitated using anti-FLAG M2 agarose beads and subjected to trypsin or chymotrypsin digestion followed by the LC-MS/MS analysis.
(B) Inhibition of LKB1 Ser325 and Ser428 phosphorylation by MEK inhibitors U0126 and PD98059. Cell lysates from SK-Mel-28 stably expressing FLAG-LKB1 were immunoprecipitated with anti-FLAG M2 agarose beads followed by western blotting using indicated antibodies. Numbers indicate relative intensity as quantified by image J analysis.
(C) Attenuation of LKB1 Ser325 and Ser428 phosphorylation upon knockdown of B-RAF expression. SK-Mel-28 cells stably expressing FLAG-LKB1 were infected with retrovirus containing two different shRNA constructs in pSUPER-retro against B-RAF or pSUPER-retro empty vector. Cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads followed by western blotting using indicated antibodies. Numbers indicate relative intensity as quantified by image J analysis.
(D) ERK directly phosphoryates LKB1 in vitro. GST-LKB1 (D194A) proteins were expressed in E. coli, purified and incubated with active recombinant ERK proteins in the presence of γ-32P-ATP. Autoradiography was performed.
(E) Ser325 is critical for phosphorylation of LKB1 by ERK in vitro. HA-LKB1 WT and S325A mutant were immunoprecipitated from HEK293 cells and incubated with recombinant ERK proteins. Protein from the assays and HEK293 cell lysates were used for western blotting analysis with phospho-S325 LKB1 antibody and HA antibody, respectively.
(F) HA-LKB1 coimmunoprecipitates with FLAG-ERK2. HEK293 cells were transfected with HA-LKB1 together with FLAG-ERK2 wildtype or kinase dead mutant. Cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads followed by western blotting with HA antibody.
(G) HA-ERK2 coimmunoprecipitates with FLAG-LKB1-N, but no FLAG-LKB1-C. Cos-7 cells were transfected with HA-ERK2 together with FLAG-LKB1 full-length (FL), N (a.a 1-309), C (a.a.310-433) or control vector. Cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads followed by western blotting with HA antibody.
(H) HA-LKB1 coimmunoprecipitates with B-RAF V600E, but not WT B-RAF. HEK293 cells were transfected with FLAG-BRAF, HA-LKB1 or empty vectors as indicated. Cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads followed by immunoblotting with indicated antibodies.
(I) Expression of BRAF V600E enhances the association between LKB1 and ERK. HEK293 cells were transfected with FLAG-LKB1, HA-ERK together with control vector, FLAG-B-RAF WT or B-RAF V600E constructs as indicated. Cell lysates were immunoprecipitated with anti-HA antibodies followed by immunoblotting with indicated antibodies.
