Abstract
The antibacterial spectrum of besifloxacin, a novel fluoroquinolone recently approved for treatment of ocular infections, was studied using 2,690 clinical isolates representing 40 species. Overall, besifloxacin was the most potent agent tested against gram-positive pathogens and anaerobes and was generally equivalent to comparator fluoroquinolones in activity against most gram-negative pathogens. Besifloxacin demonstrated potent, broad-spectrum activity, which was particularly notable against gram-positive and gram-negative isolates that were resistant to other fluoroquinolones and classes of antibacterial agents.
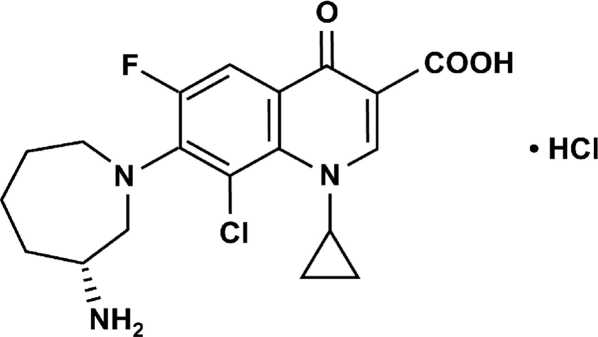
Bacterial conjunctivitis is an inflammation of the transparent mucous membrane covering the globe of the eye. This common ocular surface infection is caused by a broad variety of bacteria and is usually treated with broad-spectrum topical ophthalmic antibacterials (1, 13, 19, 20). The increasing prevalence of drug-resistant ocular isolates highlights the need for the development of new agents for the treatment of bacterial infections (8). Besifloxacin (Fig. 1) is a novel 8-chloro-fluoroquinolone agent with potent, bactericidal activity against prevalent and drug-resistant pathogens (6). Clinical development of the agent for use as a topical ophthalmic agent has been completed, and the agent has demonstrated consistent safety and efficacy in three bacterial conjunctivitis clinical trials (14, 16, 21). Pharmacokinetic studies demonstrated that after a single topical dose of 0.6% besifloxacin ophthalmic suspension, mean besifloxacin levels in human tears ranged from 610 μg/ml at 10 min postadministration to 1.6 μg/ml at 24 h postadministration (12). Here, the in vitro activity of besifloxacin against a broad range of aerobic and anaerobic bacterial species, including problematic drug-resistant strains, was evaluated.
FIG. 1.
Chemical structure of besifloxacin hydrochloride.
Totals of 2,535 aerobic and 155 anaerobic bacterial clinical isolates from Eurofins Medinet (Chantilly, VA) were selected for the study. The majority of isolates were from 2005 to 2008, and wherever possible, isolates were from ocular and respiratory specimens of U.S. origin. Susceptibility testing was conducted per Clinical and Laboratory Standards Institute reference methods (9-11).
Activity of besifloxacin against gram-positive aerobes.
Against Enterococcus faecalis and E. faecium, including vancomycin-resistant enterococci, besifloxacin was more potent than the comparator fluoroquinolones, as well as azithromycin, vancomycin, and tobramycin (Table 1). Besifloxacin further demonstrated excellent activity against Listeria monocytogenes, similar to that of tobramycin and penicillin and better than that observed with comparator fluoroquinolones.
TABLE 1.
Activities of besifloxacin and comparators against gram-positive bacteria
| Species or bacterial group (no. of isolates and phenotypea) and test drug | MIC (μg/ml)b
|
|||
|---|---|---|---|---|
| Range | 50% | 90% | %Sc | |
| Enterococcus faecalis (n = 130; 20.0% VRE) | ||||
| Besifloxacin | ≤0.03-8 | 0.12 | 2 | NA |
| Moxifloxacin | ≤0.03-32 | 0.25 | 16 | NA |
| Ciprofloxacin | 0.12->128 | 1 | 64 | 58.5 |
| Levofloxacin | 0.12-128 | 1 | 64 | 58.5 |
| Azithromycin | 0.25->128 | >128 | >128 | NA |
| Tobramycin | 2->128 | 16 | >128 | NA |
| Vancomycin | ≤0.5->32 | 1 | >32 | 80.0 |
| Enterococcus faecium (n = 110; 12.7% VRE) | ||||
| Besifloxacin | 0.06-32 | 2 | 8 | NA |
| Moxifloxacin | 0.12-64 | 16 | 64 | NA |
| Ciprofloxacin | 0.25->128 | 64 | >128 | 18.2 |
| Levofloxacin | 0.25->128 | 32 | 128 | 30.9 |
| Azithromycin | 0.06->128 | >128 | >128 | NA |
| Tobramycin | 2->128 | >128 | >128 | NA |
| Vancomycin | ≤0.5->32 | ≤0.5 | >32 | 87.3 |
| Listeria monocytogenes (n = 25) | ||||
| Besifloxacin | 0.06-0.25 | 0.12 | 0.12 | NA |
| Moxifloxacin | 0.12-0.5 | 0.25 | 0.5 | NA |
| Ciprofloxacin | 0.25-1 | 1 | 1 | NA |
| Levofloxacin | 0.25-2 | 0.5 | 1 | NA |
| Azithromycin | 0.25-1 | 0.5 | 1 | NA |
| Tobramycin | ≤0.06-0.25 | 0.12 | 0.12 | NA |
| Penicillin | ≤0.06-0.5 | 0.25 | 0.25 | 100.0 |
| Staphylococcus aureus (n = 19; CIP-S; 21.1% MRSA) | ||||
| Besifloxacin | 0.015-0.25 | 0.015 | 0.12 | NA |
| Moxifloxacin | 0.015-0.06 | 0.03 | 0.06 | 100.0 |
| Gatifloxacin | 0.03-1 | 0.06 | 0.25 | 94.7 |
| Ciprofloxacin | 0.12-0.5 | 0.25 | 0.5 | 100.0 |
| Levofloxacin | 0.06-2 | 0.12 | 0.25 | 94.7 |
| Azithromycin | 0.5->8 | 1 | >8 | 68.4 |
| Tobramycin | 0.12-8 | 0.5 | 1 | 94.7 |
| Oxacillin | 0.12->8 | 0.25 | >8 | 78.9 |
| Staphylococcus aureus (n = 11; CIP-NS; 63.6% MRSA) | ||||
| Besifloxacin | 0.03-4 | 0.5 | 4 | NA |
| Moxifloxacin | 0.06->8 | 4 | >8 | 9.1 |
| Gatifloxacin | 0.12->8 | 4 | >8 | 9.1 |
| Ciprofloxacin | 2->8 | >8 | >8 | 0.0 |
| Levofloxacin | 0.25->8 | >8 | >8 | 18.2 |
| Azithromycin | 0.5->8 | >8 | >8 | 27.3 |
| Tobramycin | 0.25->32 | 1 | >32 | 54.5 |
| Oxacillin | 0.12->8 | >8 | >8 | 36.4 |
| Staphylococcus epidermidis (n = 9; CIP-S; 44.4% MRSE) | ||||
| Besifloxacin | 0.015-0.03 | 0.03 | NA | NA |
| Moxifloxacin | 0.03-0.06 | 0.06 | NA | 100.0 |
| Gatifloxacin | 0.06-0.06 | 0.06 | NA | 100.0 |
| Ciprofloxacin | 0.12-0.12 | 0.12 | NA | 100.0 |
| Levofloxacin | 0.12-0.12 | 0.12 | NA | 100.0 |
| Azithromycin | 0.25->8 | >8 | NA | 33.3 |
| Tobramycin | ≤0.008-8 | 0.03 | NA | 88.9 |
| Oxacillin | ≤0.06-2 | 0.12 | NA | 55.6 |
| Staphylococcus epidermidis (n = 6; CIP-NS; 83.3% MRSE) | ||||
| Besifloxacin | 0.25-4 | 0.25 | NA | NA |
| Moxifloxacin | 1->8 | 2 | NA | 0.0 |
| Gatifloxacin | 1->8 | 1 | NA | 0.0 |
| Ciprofloxacin | 2->8 | >8 | NA | 0.0 |
| Levofloxacin | 2->8 | 8 | NA | 0.0 |
| Azithromycin | 0.12->8 | >8 | NA | 16.7 |
| Tobramycin | 0.06-16 | 2 | NA | 83.3 |
| Oxacillin | 0.12-4 | 1 | NA | 16.7 |
| Staphylococcus haemolyticus (n = 101) | ||||
| Besifloxacin | 0.015-4 | 0.5 | 1 | NA |
| Moxifloxacin | 0.015->8 | 1 | 8 | 39.6 |
| Gatifloxacin | 0.03->8 | 2 | 8 | 40.6 |
| Ciprofloxacin | 0.06->8 | >8 | >8 | 37.6 |
| Levofloxacin | 0.06->8 | 4 | >8 | 39.6 |
| Azithromycin | 0.25->8 | >8 | >8 | 26.7 |
| Tobramycin | 0.015->32 | 2 | 32 | 64.4 |
| Oxacillin | ≤0.06->8 | >8 | >8 | 31.7 |
| Staphylococcus hominis (n = 50) | ||||
| Besifloxacin | 0.015-2 | 0.25 | 1 | NA |
| Moxifloxacin | 0.03->8 | 1 | 4 | 34.0 |
| Gatifloxacin | 0.03->8 | 1 | 4 | 32.0 |
| Ciprofloxacin | 0.06->8 | 8 | >8 | 30.0 |
| Levofloxacin | 0.06->8 | 8 | >8 | 30.0 |
| Azithromycin | 0.12->8 | >8 | >8 | 16.0 |
| Tobramycin | 0.015->32 | 16 | 32 | 32.0 |
| Oxacillin | ≤0.06->8 | >8 | >8 | 16.0 |
| Staphylococcus lugdunensis (n = 15) | ||||
| Besifloxacin | 0.015-2 | 0.06 | 0.5 | NA |
| Moxifloxacin | 0.03->8 | 0.12 | 2 | 73.3 |
| Gatifloxacin | 0.03-8 | 0.12 | 2 | 73.3 |
| Ciprofloxacin | 0.06->8 | 0.12 | >8 | 66.7 |
| Levofloxacin | 0.06->8 | 0.25 | >8 | 66.7 |
| Azithromycin | 0.25->8 | >8 | >8 | 46.7 |
| Tobramycin | 0.03->32 | 0.12 | 32 | 60.0 |
| Oxacillin | ≤0.06->8 | 0.5 | >8 | 60.0 |
| Staphylococcus saprophyticus (n = 101) | ||||
| Besifloxacin | 0.015-0.25 | 0.06 | 0.12 | NA |
| Moxifloxacin | 0.03-0.25 | 0.12 | 0.12 | 100.0 |
| Gatifloxacin | 0.03-0.25 | 0.12 | 0.25 | 100.0 |
| Levofloxacin | 0.06-0.5 | 0.5 | 0.5 | 100.0 |
| Ciprofloxacin | 0.06-0.5 | 0.25 | 0.5 | 100.0 |
| Azithromycin | 0.12->8 | 1 | >8 | 54.5 |
| Tobramycin | ≤0.008-32 | 0.015 | 0.06 | 99.0 |
| Oxacillin | ≤0.06->8 | 0.5 | 1 | 9.9 |
| Staphylococcus warneri (n = 50) | ||||
| Besifloxacin | 0.015-2 | 0.06 | 1 | NA |
| Moxifloxacin | 0.015->8 | 0.06 | 4 | 76.0 |
| Gatifloxacin | 0.03->8 | 0.12 | 4 | 76.0 |
| Ciprofloxacin | 0.06->8 | 0.25 | >8 | 74.0 |
| Levofloxacin | 0.06->8 | 0.12 | >8 | 76.0 |
| Azithromycin | 0.12->8 | >8 | >8 | 34.0 |
| Tobramycin | 0.015->32 | 0.06 | 8 | 86.0 |
| Oxacillin | ≤0.06->8 | 0.5 | >8 | 46.0 |
| Streptococcus agalactiae (n = 100) | ||||
| Besifloxacin | 0.03-0.12 | 0.06 | 0.06 | NA |
| Moxifloxacin | 0.06-1 | 0.12 | 0.25 | NA |
| Gatifloxacin | 0.12-1 | 0.25 | 0.25 | 100.0 |
| Ciprofloxacin | 0.5-8 | 0.5 | 1 | NA |
| Levofloxacin | 0.25-4 | 0.5 | 1 | 98.0 |
| Azithromycin | 0.015->8 | 0.06 | >8 | 73.0 |
| Tobramycin | 8->128 | 32 | 64 | NA |
| Penicillin | ≤0.015-0.06 | 0.03 | 0.06 | 100.0 |
| Lancefield group C, F, and G streptococci (n = 50) | ||||
| Besifloxacin | 0.015-0.25 | 0.03 | 0.06 | NA |
| Moxifloxacin | 0.03-1 | 0.12 | 0.12 | NA |
| Gatifloxacin | 0.06-2 | 0.12 | 0.25 | 98.0 |
| Ciprofloxacin | 0.12->8 | 0.5 | 0.5 | NA |
| Levofloxacin | 0.12-8 | 0.5 | 0.5 | 98.0 |
| Azithromycin | 0.008->8 | 0.06 | >8 | 74.0 |
| Tobramycin | 2-32 | 8 | 16 | NA |
| Penicillin | ≤0.015-0.06 | ≤0.015 | 0.06 | 100.0 |
| Streptococcus pneumoniae (n = 16; LVX-S) | ||||
| Besifloxacin | 0.015-0.25 | 0.03 | 0.06 | NA |
| Moxifloxacin | 0.03-1 | 0.06 | 0.12 | 100.0 |
| Ciprofloxacin | 0.12-8 | 0.5 | 2 | 93.8 |
| Levofloxacin | 0.25-2 | 0.5 | 0.5 | 100 |
| Azithromycin | ≤0.03->64 | 0.06 | >64 | 62.5 |
| Tobramycin | 4-32 | 8 | 16 | NA |
| Penicillin | ≤0.06-2 | ≤0.06 | 0.5 | 100.0 |
| Streptococcus pneumoniae (n = 85; LVX-NS) | ||||
| Besifloxacin | 0.008-1 | 0.5 | 0.5 | NA |
| Moxifloxacin | 0.25-8 | 2 | 4 | 16.5 |
| Ciprofloxacin | 1-64 | 32 | 64 | 1.2 |
| Levofloxacin | 4-32 | 16 | 16 | 0 |
| Azithromycin | ≤0.03->64 | 4 | >64 | 36.5 |
| Tobramycin | 1-32 | 8 | 16 | NA |
| Penicillin | ≤0.06-2 | 0.12 | 2 | 100.0 |
| Streptococcus pyogenes (n = 101) | ||||
| Besifloxacin | 0.03-0.06 | 0.03 | 0.06 | NA |
| Moxifloxacin | 0.06-0.5 | 0.12 | 0.25 | NA |
| Gatifloxacin | 0.06-0.5 | 0.12 | 0.25 | 100.0 |
| Ciprofloxacin | 0.12-2 | 0.5 | 0.5 | NA |
| Levofloxacin | 0.25-2 | 0.5 | 0.5 | 100.0 |
| Azithromycin | 0.03->8 | 0.06 | 8 | 85.1 |
| Tobramycin | 4-64 | 16 | 16 | NA |
| Penicillin | ≤0.015-0.06 | ≤0.015 | ≤0.015 | 100.0 |
| Viridans streptococcid (n = 156) | ||||
| Besifloxacin | 0.015-2 | 0.06 | 0.12 | NA |
| Moxifloxacin | 0.03-4 | 0.12 | 0.25 | NA |
| Gatifloxacin | 0.03-8 | 0.25 | 0.5 | NA |
| Ciprofloxacin | 0.12->8 | 1 | 4 | NA |
| Levofloxacin | 0.12->8 | 1 | 1 | 95.5 |
| Azithromycin | 0.008->8 | 0.06 | >8 | 53.2 |
| Tobramycin | 0.5-128 | 16 | 32 | NA |
| Penicillin | ≤0.015->4 | 0.06 | 1 | 76.3 |
VRE, vancomycin-intermediate and -resistant enterococci; MRSE, methicillin-resistant S. epidermidis; CIP-S, ciprofloxacin susceptible; CIP-NS, non-ciprofloxacin susceptible; LVX-S, levofloxacin susceptible; LVX-NS, non-levofloxacin susceptible.
NA, not applicable.
Percentage of susceptible isolates (9); breakpoints have not been defined for all agent-species combinations.
The viridans streptococcal group consisted of 2 Streptococcus anginosus, 13 S. bovis, 7 S. constellatus, 28 S. intermedius, 51 S. mitis, 22 S. oralis, 2 S. salivarius, 17 S. sanguinis, and 14 other viridans group isolates.
For Staphylococcus aureus and S. epidermidis, previously reported MIC50s/MIC90s were consistent with current besifloxacin values for quinolone-susceptible and quinolone-resistant subsets (3, 5, 14). Besifloxacin was especially potent against ciprofloxacin-resistant isolates; for example, the MIC90s for non-ciprofloxacin-susceptible S. aureus were 4 μg/ml for besifloxacin but out of range (>8 μg/ml) for all other fluoroquinolones. A similar trend was noted for S. epidermidis. As indicated in Table 1, 21.1% to 83.3% of the S. aureus and S. epidermidis ocular isolates tested were also methicillin (meticillin) resistant; however, resistance to methicillin did not affect the susceptibility to besifloxacin (data not shown). Against five other staphylococcal species, besifloxacin was generally the most active fluoroquinolone tested, even in instances where the test group contained a significant fraction of isolates that were not susceptible to the comparator agents. For staphylococci overall, besifloxacin was the most active agent tested.
Besifloxacin also demonstrated potent activity against a broad range of streptococci, including Streptococcus agalactiae, Lancefield group C, F, and G streptococci, S. pneumoniae, S. pyogenes, and viridans streptococci. For S. pneumoniae, besifloxacin MIC90s were 0.06 μg/ml for levofloxacin-susceptible isolates and 0.5 μg/ml for non-levofloxacin-susceptible isolates (Table 1). Comparator fluoroquinolones had MIC90s that were two- and eightfold higher, respectively. These results were similar to those from previous studies that reported overall S. pneumoniae besifloxacin MIC90s of ≤0.12 μg/ml and showed that penicillin-resistant strains were equally susceptible to besifloxacin (5, 15). For all other streptococci tested, which included up to 46.8% azithromycin-resistant isolates, besifloxacin MIC90s were 2- to 16-fold lower than those observed for other fluoroquinolones. In general, the activity of besifloxacin against streptococci was greater than that of the comparator fluoroquinolones as well as that of azithromycin and tobramycin.
Activity of besifloxacin against gram-negative aerobes.
For three Acinetobacter spp. (which included 16 to 64% non-ciprofloxacin-susceptible isolates), the besifloxacin MIC90s (16 to 32 μg/ml) were similar to or lower than the ciprofloxacin values, and for two of these species, the besifloxacin MIC90s were two- to fourfold higher than the corresponding values for levofloxacin and moxifloxacin (Table 2).
TABLE 2.
Activities of besifloxacin and comparators against gram-negative bacteria
| Species (no. of isolates and phenotypea) and test drug | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Range | 50% | 90% | %Sb | |
| Acinetobacter baumanii (n = 53) | ||||
| Besifloxacin | 0.015-64 | 16 | 32 | NA |
| Moxifloxacin | 0.03-128 | 8 | 32 | NA |
| Ciprofloxacin | 0.06->128 | 64 | >128 | 35.8 |
| Levofloxacin | 0.03-128 | 8 | 64 | 37.7 |
| Imipenem | 0.12->8 | 2 | >8 | 54.7 |
| Tobramycin | 0.12->64 | 4 | >64 | 60.4 |
| Ceftazidime | 2->16 | >16 | >16 | 39.6 |
| Acinetobacter calcoaceticus (n = 27) | ||||
| Besifloxacin | 0.06-32 | 0.5 | 16 | NA |
| Moxifloxacin | 0.015-128 | 2 | 8 | NA |
| Ciprofloxacin | 0.03->128 | 1 | 128 | 51.9 |
| Levofloxacin | 0.015-128 | 0.5 | 8 | 66.7 |
| Imipenem | 0.06->8 | 0.25 | 1 | 92.6 |
| Tobramycin | 0.12->64 | 1 | >64 | 77.8 |
| Ceftazidime | 2->16 | 4 | >16 | 63.0 |
| Acinetobacter lwoffii (n = 50) | ||||
| Besifloxacin | 0.015-64 | 0.25 | 16 | NA |
| Moxifloxacin | 0.008-128 | 0.06 | 4 | NA |
| Ciprofloxacin | 0.004->128 | 0.12 | 16 | 84.0 |
| Levofloxacin | 0.015-64 | 0.12 | 4 | 86.0 |
| Imipenem | ≤0.03->8 | 0.06 | 0.5 | 94.0 |
| Tobramycin | ≤0.06->64 | 0.25 | 8 | 86.0 |
| Ceftazidime | 0.5->16 | 2 | >16 | 76.0 |
| Citrobacter freundii (n = 100) | ||||
| Besifloxacin | 0.015-32 | 0.25 | 4 | NA |
| Moxifloxacin | 0.03-32 | 0.12 | 4 | 85.0 |
| Ciprofloxacin | 0.004-64 | 0.03 | 2 | 87.0 |
| Levofloxacin | 0.015-32 | 0.06 | 2 | 90.0 |
| Imipenem | ≤0.03->8 | 0.25 | 0.5 | 99.0 |
| Tobramycin | 0.12->64 | 0.5 | 8 | 87.0 |
| Ceftazidime | 0.06->16 | 0.25 | >16 | 78.0 |
| Citrobacter koseri (n = 100) | ||||
| Besifloxacin | 0.03->8 | 0.06 | 0.25 | NA |
| Moxifloxacin | 0.015->8 | 0.03 | 0.25 | NA |
| Gatifloxacin | 0.008->8 | 0.015 | 0.12 | 99.0 |
| Ciprofloxacin | 0.004->8 | 0.008 | 0.06 | 99.0 |
| Levofloxacin | 0.015->8 | 0.03 | 0.12 | 99.0 |
| Azithromycin | 2->8 | 8 | >8 | NA |
| Tobramycin | 0.25-16 | 0.5 | 1 | 99.0 |
| Ceftazidime | 0.06-4 | 0.12 | 0.5 | 100.0 |
| Enterobacter aerogenes (n = 50) | ||||
| Besifloxacin | 0.03-16 | 0.25 | 8 | NA |
| Moxifloxacin | 0.03-64 | 0.12 | 16 | 80.0 |
| Ciprofloxacin | 0.008-64 | 0.015 | 16 | 80.0 |
| Levofloxacin | 0.03-64 | 0.06 | 16 | 80.0 |
| Imipenem | 0.12-4 | 0.5 | 1 | 100.0 |
| Tobramycin | 0.25-32 | 0.5 | 8 | 88.0 |
| Ceftazidime | 0.12->16 | 2 | >16 | 62.0 |
| Enterobacter cloacae (n = 50) | ||||
| Besifloxacin | 0.06-16 | 0.12 | 2 | NA |
| Moxifloxacin | 0.015-16 | 0.06 | 2 | 94.0 |
| Ciprofloxacin | 0.004-64 | 0.015 | 0.5 | 92.0 |
| Levofloxacin | 0.008-32 | 0.03 | 0.25 | 92.0 |
| Imipenem | 0.12-2 | 0.25 | 1 | 100.0 |
| Tobramycin | 0.25-32 | 0.5 | 8 | 88.0 |
| Ceftazidime | 0.12->16 | 0.5 | >16 | 70.0 |
| Haemophilus influenzae (n = 15; CIP-S) | ||||
| Besifloxacin | 0.008-0.25 | 0.015 | 0.03 | NA |
| Moxifloxacin | 0.015-0.5 | 0.015 | 0.06 | 100.0 |
| Ciprofloxacin | 0.008-1 | 0.008 | 0.015 | 100.0 |
| Levofloxacin | 0.008-1 | 0.015 | 0.015 | 100.0 |
| Azithromycin | 0.12-2 | 2 | 2 | 100.0 |
| Tobramycin | 1-16 | 2 | 8 | NA |
| Penicillin | 0.25->4 | 0.5 | >4 | NA |
| Haemophilus influenzae (n = 11; CIP-NS) | ||||
| Besifloxacin | 0.015-2 | 2 | 2 | NA |
| Moxifloxacin | 1-16 | 8 | 16 | 9.1 |
| Ciprofloxacin | 2-64 | 16 | 64 | 0 |
| Levofloxacin | 1-32 | 8 | 16 | 27.3 |
| Azithromycin | 0.5-8 | 1 | 8 | 81.8 |
| Tobramycin | 1-16 | 4 | 8 | NA |
| Penicillin | 0.5->4 | 1 | 2 | NA |
| Klebsiella oxytoca (n = 50) | ||||
| Besifloxacin | 0.06-8 | 0.12 | 1 | NA |
| Moxifloxacin | 0.03-8 | 0.06 | 2 | NA |
| Gatifloxacin | 0.015-8 | 0.03 | 0.5 | 92.0 |
| Ciprofloxacin | 0.008->8 | 0.015 | 0.5 | 90.0 |
| Levofloxacin | 0.015-8 | 0.03 | 0.5 | 90.0 |
| Azithromycin | 8->8 | >8 | >8 | NA |
| Tobramycin | 0.25-8 | 0.5 | 1 | 96.0 |
| Ceftazidime | 0.03-1 | 0.12 | 0.5 | 100.0 |
| Klebsiella pneumoniae (n = 100) | ||||
| Besifloxacin | ≤0.004-64 | 0.25 | 4 | NA |
| Moxifloxacin | 0.03-128 | 0.12 | 2 | 90.0 |
| Ciprofloxacin | 0.008->128 | 0.03 | 2 | 89.0 |
| Levofloxacin | ≤0.004-128 | 0.06 | 2 | 91.0 |
| Imipenem | ≤0.03-2 | 0.25 | 0.5 | 100.0 |
| Tobramycin | 0.12->64 | 0.25 | 1 | 93.0 |
| Ceftazidime | ≤0.03->16 | 0.12 | 1 | 92.0 |
| Legionella pneumophila (n = 50) | ||||
| Besifloxacin | 0.015-0.06 | 0.03 | 0.03 | NA |
| Moxifloxacin | 0.015-0.06 | 0.03 | 0.06 | NA |
| Gatifloxacin | 0.015-0.06 | 0.03 | 0.06 | NA |
| Ciprofloxacin | 0.015-0.06 | 0.03 | 0.03 | NA |
| Levofloxacin | 0.015-0.06 | 0.03 | 0.03 | NA |
| Azithromycin | 0.03-1 | 0.12 | 1 | NA |
| Tobramycin | 0.25-4 | 1 | 2 | NA |
| Moraxella catarrhalis (n = 101) | ||||
| Besifloxacin | 0.015-0.12 | 0.03 | 0.03 | NA |
| Moxifloxacin | 0.015-0.12 | 0.03 | 0.03 | NA |
| Gatifloxacin | 0.008-0.25 | 0.015 | 0.015 | NA |
| Ciprofloxacin | 0.008-0.25 | 0.015 | 0.015 | 100.0 |
| Levofloxacin | 0.015-0.5 | 0.015 | 0.03 | 100.0 |
| Azithromycin | 0.015-0.06 | 0.03 | 0.03 | 100.0 |
| Tobramycin | 0.03-0.5 | 0.25 | 0.25 | NA |
| Oxacillin | 0.25->8 | 4 | 8 | NA |
| Morganella morganii (n = 51) | ||||
| Besifloxacin | 0.03->8 | 0.12 | 4 | NA |
| Moxifloxacin | 0.03->8 | 0.25 | >8 | NA |
| Gatifloxacin | 0.015->8 | 0.12 | >8 | 74.5 |
| Ciprofloxacin | 0.004->8 | 0.015 | >8 | 76.5 |
| Levofloxacin | 0.015->8 | 0.06 | 8 | 76.5 |
| Azithromycin | 8->8 | >8 | >8 | NA |
| Tobramycin | 0.25-32 | 1 | 4 | 90.2 |
| Ceftazidime | 0.03->32 | 0.12 | 16 | 82.4 |
| Neisseria meningitidis (n = 20) | ||||
| Besifloxacin | ≤0.004-0.03 | 0.008 | 0.015 | NA |
| Moxifloxacin | ≤0.004-0.015 | 0.008 | 0.008 | NA |
| Ciprofloxacin | ≤0.002-0.015 | 0.004 | 0.004 | 100.0 |
| Levofloxacin | ≤0.004-0.03 | 0.008 | 0.015 | 100.0 |
| Azithromycin | ≤0.03-0.12 | 0.06 | 0.06 | 100.0 |
| Penicillin | ≤0.06-0.25 | ≤0.06 | 0.12 | 50.0 |
| Proteus mirabilis (n = 50) | ||||
| Besifloxacin | 0.06-32 | 0.25 | 16 | NA |
| Moxifloxacin | 0.06-128 | 0.25 | 16 | 62.0 |
| Ciprofloxacin | 0.015->128 | 0.03 | 32 | 64.0 |
| Levofloxacin | 0.015-32 | 0.03 | 8 | 76.0 |
| Imipenem | 0.06-4 | 1 | 2 | 100.0 |
| Tobramycin | 0.25-32 | 1 | 4 | 92.0 |
| Ceftazidime | ≤0.03-16 | ≤0.03 | 0.06 | 98.0 |
| Proteus vulgaris (n = 50) | ||||
| Besifloxacin | 0.06-1 | 0.12 | 0.25 | NA |
| Moxifloxacin | 0.06-2 | 0.25 | 0.5 | 100.0 |
| Ciprofloxacin | 0.008-0.12 | 0.015 | 0.03 | 100.0 |
| Levofloxacin | 0.015-0.25 | 0.03 | 0.06 | 100.0 |
| Imipenem | 0.25-4 | 1 | 4 | 100.0 |
| Tobramycin | 0.25-64 | 1 | 2 | 98.0 |
| Ceftazidime | ≤0.03-2 | 0.06 | 0.12 | 100.0 |
| Pseudomonas aeruginosa (n = 105; CIP-S) | ||||
| Besifloxacin | 0.5-8 | 1 | 4 | NA |
| Moxifloxacin | 0.5-16 | 2 | 4 | NA |
| Ciprofloxacin | 0.03-1 | 0.12 | 0.5 | 100.0 |
| Levofloxacin | 0.06-4 | 0.5 | 1 | 96.2 |
| Imipenem | 0.5->8 | 2 | 2 | 95.2 |
| Tobramycin | 0.25->64 | 0.5 | 1 | 97.1 |
| Ceftazidime | 0.5->16 | 2 | 8 | 93.3 |
| Pseudomonas aeruginosa (n = 96; CIP-NS) | ||||
| Besifloxacin | 2-128 | 16 | 64 | NA |
| Moxifloxacin | 2->128 | 64 | >128 | NA |
| Ciprofloxacin | 2->128 | 16 | 64 | 0 |
| Levofloxacin | 2->128 | 16 | 64 | 1.0 |
| Imipenem | 0.25->8 | 8 | >8 | 44.8 |
| Tobramycin | 0.25->64 | 1 | >64 | 64.6 |
| Ceftazidime | 0.25->16 | 4 | >16 | 57.3 |
| Serratia marcescens (n = 100) | ||||
| Besifloxacin | 0.12-2 | 0.25 | 1 | NA |
| Moxifloxacin | 0.06-4 | 0.25 | 1 | 98.0 |
| Ciprofloxacin | 0.015-2 | 0.06 | 0.5 | 98.0 |
| Levofloxacin | 0.03-1 | 0.12 | 0.5 | 100.0 |
| Imipenem | 0.12-2 | 0.5 | 1 | 100.0 |
| Tobramycin | 0.25->64 | 2 | 4 | 91.0 |
| Ceftazidime | ≤0.03->16 | 0.06 | 0.5 | 93.0 |
| Stenotrophomonas maltophilia (n = 48; LVX-S) | ||||
| Besifloxacin | 0.25-8 | 1 | 4 | NA |
| Moxifloxacin | 0.03-1 | 0.5 | 1 | NA |
| Ciprofloxacin | 0.5-8 | 2 | 4 | 33.3 |
| Levofloxacin | 0.12-2 | 1 | 2 | 100.0 |
| Imipenem | >8 | >8 | >8 | 0 |
| Tobramycin | 1->64 | 16 | >64 | 22.9 |
| Ceftazidime | 0.5->16 | 16 | >16 | 43.8 |
| Stenotrophomonas maltophilia (LVX-NS; n = 52) | ||||
| Besifloxacin | 1->128 | 16 | 64 | NA |
| Moxifloxacin | 0.5-64 | 8 | 32 | NA |
| Ciprofloxacin | 4-128 | 32 | 64 | 0 |
| Levofloxacin | 4-64 | 8 | 32 | 0 |
| Imipenem | 2->8 | >8 | >8 | 1.9 |
| Tobramycin | 0.25->64 | 16 | >64 | 38.5 |
| Ceftazidime | 0.5->16 | >16 | >16 | 32.7 |
CIP-S, ciprofloxacin susceptible; CIP-NS, non-ciprofloxacin susceptible; LVX-S, levofloxacin susceptible; LVX-NS, non-levofloxacin susceptible.
Percentage of susceptible isolates (9); breakpoints have not been defined for all agent-species combinations. NA, not applicable.
For the Enterobacteriaceae, besifloxacin MIC90s were identical to or lower than those of moxifloxacin for all species but K. pneumoniae (Table 2), where the besifloxacin MIC90 was twofold higher than that of moxifloxacin (4 versus 2 μg/ml, respectively). Ciprofloxacin and levofloxacin were generally the most active fluoroquinolones tested against the Enterobacteriaceae. However, a comparison of MIC90s for species that included over 20% non-ciprofloxacin-susceptible isolates (Enterobacter aerogenes, Morganella morganii, and Proteus mirabilis) indicates that besifloxacin is especially active against fluoroquinolone-resistant isolates.
For the fastidious gram-negative species (Haemophilus influenzae, Moraxella catarrhalis, Neisseria meningitidis, and Legionella pneumophila), besifloxacin demonstrated potent activity equivalent to that of the fluoroquinolone comparators, with MIC90s of 0.03 μg/ml or less for all test groups except non-ciprofloxacin-susceptible H. influenzae (Table 2). Results from previous studies (4, 5, 17) were consistent with the current results, with all studies yielding besifloxacin MIC90s of 0.06 μg/ml or less for ciprofloxacin-susceptible H. influenzae. For non-ciprofloxacin-susceptible isolates of H. influenzae, besifloxacin was 8- to 32-fold more active than the other fluoroquinolones.
Against ciprofloxacin-susceptible Pseudomonas aeruginosa, ciprofloxacin and levofloxacin were the most active fluoroquinolones (MIC90s of 0.5 to 1 μg/ml), while both besifloxacin and moxifloxacin showed MIC90s of 4 μg/ml (Table 2). However, against non-ciprofloxacin-susceptible P. aeruginosa, MIC90s for besifloxacin, levofloxacin, and ciprofloxacin were 64 μg/ml, whereas the corresponding moxifloxacin MIC90 was at least fourfold higher (>128 μg/ml). For levofloxacin-susceptible and non-levofloxacin-susceptible Stenotrophomonas maltophilia isolates, the besifloxacin MIC90s (4 and 64 μg/ml, respectively) were identical to those for ciprofloxacin and two- to fourfold higher than those for levofloxacin or moxifloxacin.
The overall activity of besifloxacin against gram-negative aerobic pathogens was generally similar to or two- to fourfold less than that of the other fluoroquinolones tested against fluoroquinolone-susceptible isolates. However, against non-ciprofloxacin-susceptible isolates, besifloxacin was more potent than or equal to the comparator fluoroquinolones.
Activity of besifloxacin against gram-positive and -negative anaerobes.
For five of the six anaerobic species, besifloxacin MIC90s were equal to or lower than those for the most active comparators, including clindamycin, metronidazole, and the other fluoroquinolones (Table 3). Against Propionibacterium acnes, the most active agent was clindamycin, with a 0.12 μg/ml MIC90, while the corresponding besifloxacin and moxifloxacin values were twofold higher. Overall, besifloxacin and moxifloxacin were the most active agents tested against anaerobic bacteria.
TABLE 3.
Activities of besifloxacin and comparators against gram-positive and -negative anaerobic bacteria
| Species (no. of isolates) and test drug | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Range | 50% | 90% | %Sa | |
| Bacteroides fragilis (n = 20) | ||||
| Besifloxacin | 0.25-2 | 0.5 | 1 | NA |
| Moxifloxacin | 0.25-8 | 0.5 | 2 | 95.0 |
| Gatifloxacin | 1-16 | 2 | 4 | NA |
| Clindamycin | 0.5->8 | 2 | >8 | 65.0 |
| Metronidazole | 2-2 | 2 | 2 | 100 |
| Clostridium perfringens (n = 21) | ||||
| Besifloxacin | 0.12-0.25 | 0.25 | 0.25 | NA |
| Moxifloxacin | 0.25-0.5 | 0.5 | 0.5 | 100.0 |
| Gatifloxacin | 0.5-1 | 1 | 1 | NA |
| Clindamycin | 0.06-4 | 2 | 4 | 85.7 |
| Metronidazole | 1-4 | 2 | 4 | 100.0 |
| Fusobacterium spp. (n = 21) | ||||
| Besifloxacin | 0.12-8 | 0.25 | 1 | NA |
| Moxifloxacin | 0.25->16 | 1 | 2 | 95.2 |
| Gatifloxacin | 0.5->16 | 1 | 4 | NA |
| Clindamycin | 0.06-8 | 0.06 | 2 | 95.2 |
| Metronidazole | ≤0.12-2 | 0.25 | 1 | 100 |
| Peptostreptococcus spp. (n = 52) | ||||
| Besifloxacin | 0.06-2 | 0.25 | 0.5 | NA |
| Moxifloxacin | 0.25-4 | 0.25 | 0.5 | 98.1 |
| Ciprofloxacin | 0.5->8 | 2 | 4 | NA |
| Clindamycin | 0.06->8 | 0.25 | >8 | 88.5 |
| Metronidazole | ≤0.03->16 | 0.5 | 1 | 98.1 |
| Prevotella spp. (n = 20) | ||||
| Besifloxacin | 0.06-16 | 1 | 4 | NA |
| Moxifloxacin | 0.12->16 | 4 | 8 | 45.0 |
| Gatifloxacin | 0.25->16 | 8 | 16 | NA |
| Clindamycin | ≤0.03->8 | ≤0.03 | >8 | 85.0 |
| Metronidazole | 0.25-8 | 4 | 4 | 100.0 |
| Propionibacterium acnes (n = 21) | ||||
| Besifloxacin | 0.12-0.25 | 0.25 | 0.25 | NA |
| Moxifloxacin | 0.25-0.25 | 0.25 | 0.25 | 100.0 |
| Gatifloxacin | 0.25-0.5 | 0.25 | 0.5 | NA |
| Clindamycin | ≤0.03-2 | 0.06 | 0.12 | 100.0 |
| Metronidazole | >16->16 | >16 | >16 | 0 |
Percentage of susceptible isolates (10); breakpoints have not been defined for all agent-species combinations. NA, not applicable.
Besifloxacin and drug-resistant bacteria.
Recent results from the ocular TRUST project (2) indicated that 85% of methicillin-resistant S. aureus (MRSA) isolates are also resistant to fluoroquinolones. The improved potency of besifloxacin relative to that of the other fluoroquinolones as well as azithromycin and tobramycin against MRSA and non-ciprofloxacin-susceptible staphylococci should extend the agent's coverage. Although resistance to existing fluoroquinolones is at present still relatively rare among ocular S. pneumoniae and H. influenzae (2), the data presented here demonstrate that besifloxacin shows improved in vitro activity against fluoroquinolone-resistant isolates of these two major pathogens as well.
Previous genetic and biochemical studies demonstrated relatively balanced dual targeting of DNA gyrase and topoisomerase IV and lower in vitro spontaneous resistance rates for besifloxacin (7). Consistent with those results, the current study shows that for multiple gram-negative and gram-positive species, the differences in MIC between fluoroquinolone-susceptible and -resistant strains were less for besifloxacin than for the other members of this drug family. Further studies are warranted to more fully characterize how the potentially unique interactions between besifloxacin and both enzyme targets (7) may be related to the improved activity, reported herein, against a broad spectrum of clinical isolates that are resistant to earlier fluoroquinolones.
Conclusions.
Against 2,690 isolates, representing 34 aerobic and 6 anaerobic bacterial species, the in vitro activity of besifloxacin was generally equivalent or superior to that of existing agents used for topical treatment of ocular infections. The consistently improved activity profile of besifloxacin against gram-positive and gram-negative pathogens that were resistant to other fluoroquinolones was particularly notable. In conjunction with recently reported safety, efficacy, and pharmacokinetic results from clinical trials (14, 16, 18, 21), besifloxacin's broad-spectrum activity profile is appropriate for empirical treatment of bacterial infections.
Acknowledgments
This work was supported by research grants from Bausch & Lomb, Rochester, NY.
Footnotes
Published ahead of print on 8 June 2009.
REFERENCES
- 1.American Academy of Ophthalmology. Conjunctivitis, preferred practice pattern. 2003. American Academy of Ophthalmology, San Francisco, CA.
- 2.Asbell, P. A., K. A. Colby, S. Deng, P. McDonnell, D. M. Meisler, M. B. Raizman, J. D. Sheppard, Jr., and D. F. Sahm. 2008. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am. J. Ophthalmol. 145:951-958. [DOI] [PubMed] [Google Scholar]
- 3.Blondeau, J. M., L. Brunner, and S. Norton. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-2001.
- 4.Blondeau, J. M., L. Brunner, and S. Norton. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-2002.
- 5.Brunner, L., S. Norton, and J. M. Blondeau. 2007. Abstr. 17th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. P1679.
- 6.Brunner, L., P. A. Walsh, S. Norton, and J. M. Blondeau. 2007. Abstr. 17th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. P1680.
- 7.Cambau, E., S. Matrat, X. S. Pan, R. Roth Dit Bettoni, C. Corbel, A. Aubry, C. Lascols, J. Y. Driot, and L. M. Fisher. 2009. Target specificity of the new fluoroquinolone besifloxacin in Streptococcus pneumoniae, Staphylococcus aureus and Escherichia coli. J. Antimicrob. Chemother. 63:443-450. [DOI] [PubMed] [Google Scholar]
- 8.Cavuoto, K., D. Zutshi, C. L. Karp, D. Miller, and W. Feuer. 2008. Update on bacterial conjunctivitis in South Florida. Ophthalmology 115:51-56. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 7th ed. CLSI M7-A7. ISBN 1-56238-587-9. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.Clinical and Laboratory Standards Institute. 2007. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved Standard, 7th ed. CLSI M11-A7. ISBN 1-56238-626-3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 11.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. CLSI M100-S18. ISBN 1-56238-653-0. Clinical and Laboratory Standards Institute, Wayne, PA.
- 12.Granville, C. P., R. Siou-Mermet, T. Comstock, M. Jonasse, and J. W. Proksch. 2008. Abstr. Annu. Meet. Assoc. Res. Vis. Ophthalmol., abstr. D691.
- 13.Hovding, G. 2008. Acute bacterial conjunctivitis. Acta Ophthalmol. 86:5-17. [DOI] [PubMed] [Google Scholar]
- 14.Karpecki, P., M. DePaolis, J. A. Hunter, E. M. White, L. Rigel, L. S. Brunner, D. W. Usner, M. R. Paterno, and T. L. Comstock. 2009. Clinical and microbial efficacy of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Clin. Ther. 31:514-526. [DOI] [PubMed] [Google Scholar]
- 15.McDonald, M. B., J. M. Blondeau, H. H. DeCory, D. W. Usner, L. Brunner, W. Haas, and T. W. Morris. 2008. Abstr. Annu. Meet. Am. Acad. Ophthalmol., abstr. PO070.
- 16.McDonald, M. B., E. E. Protzko, L. S. Brunner, T. W. Morris, W. Haas, M. R. Paterno, T. L. Comstock, and D. W. Usner. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitis. Ophthalmology, in press. [DOI] [PubMed]
- 17.Paterno, M. R., L. Brunner, S. Norton, and J. M. Blondeau. 2007. Abstr. Annu. Meet. Assoc. Res. Vis. Ophthalmol., abstr. B541.
- 18.Proksch, J. W., C. P. Granvil, R. Mermet-Siou, T. Comstock, M. R. Paterno, and K. W. Ward. Ocular pharmacokinetics of besifloxacin following topical administration to rabbits, monkeys, and humans. J. Ocul. Pharmacol. Ther., in press. [DOI] [PubMed]
- 19.Sheikh, A., and B. Hurwitz. 2006. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst. Rev. 2006:CD001211. [DOI] [PubMed] [Google Scholar]
- 20.Tarabishy, A. B., and B. H. Jeng. 2008. Bacterial conjunctivitis: a review for internists. Cleve. Clin. J. Med. 75:507-512. [DOI] [PubMed] [Google Scholar]
- 21.Tepedino, M. E., W. H. Heller, D. W. Usner, L. S. Brunner, T. W. Morris, W. Haas, M. R. Paterno, and T. Comstock. 2009. Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Curr. Med. Res. Opin. 25:1159-1169. [DOI] [PubMed] [Google Scholar]