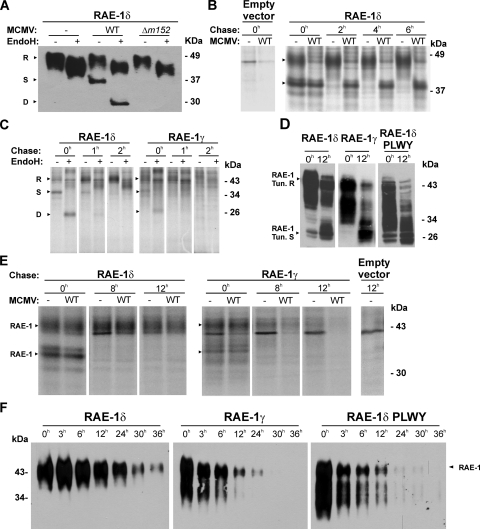
FIG. 5.
Differential stability of RAE-1δ and RAE-1γ mature forms. (A) RAE-1δ-transfected NIH 3T3 cells were infected with four PFU of WT MCMV or Δm152 MCMV/cell or left uninfected. RAE-1δ was immunoblotted from EndoH-treated or untreated lysates with anti-RAE-1δ MAbs. (B) RAE-1δ- or empty vector-transfected NIH 3T3 cells were infected with four PFU of WT MCMV or left uninfected. Cells were metabolically labeled with 300 μCi of [35S]methionine/ml 6 h to 8 h after infection and chased for the indicated periods of time, and immunoprecipitation was performed using anti-RAE-1δ MAbs, followed by protein G-Sepharose. (C) RAE-1δ- or RAE-1γ-transfected NIH 3T3 cells were labeled with 500 μCi of [35S]methionine/ml for 1 h and chased for the indicated periods of time. After immunoprecipitation with either anti-RAE-1δ or anti-RAE-1γ MAbs followed by protein G-Sepharose, eluted proteins were treated with EndoH or left untreated. (D) RAE-1γ-, RAE-1δ-, and RAE-1δ-PLWY-transfected NIH 3T3 cells were untreated or treated for 12 h with tunicamycin (2 μg/ml). Cell lysates were immunoblotted using specific anti-RAE-1 antibodies. (E) RAE-1δ-, RAE-1γ-, or empty vector-transfected NIH 3T3 cells were metabolically labeled with 300 μCi of [35S]methionine/ml for 2 h before infection with four PFU of WT MCMV. Cells were chased for indicated periods of time and immunoprecipitation was performed with either anti-RAE-1δ MAbs or anti-RAE-1γ antibodies followed by protein G-Sepharose. (F) RAE-1δ-, RAE-1γ-, and RAE-1δ-PLWY-transfected NIH 3T3 cells were surface biotinylated. After the indicated periods of time, RAE-1 molecules were immunoprecipitated using anti-FLAG M2-Sepharose, followed by immunoblotting with SA-POD. Arrows indicate different maturation forms of RAE-1 proteins: R, resistant to EndoH; S, sensitive to EndoH; D, digested with EndoH. RAE-1 Tun.R indicates the matured form of RAE-1 which is resistant to tunicamycin, whereas RAE-1 Tun.S indicates the deglycosylated form of RAE-1 proteins by tunicamycin.