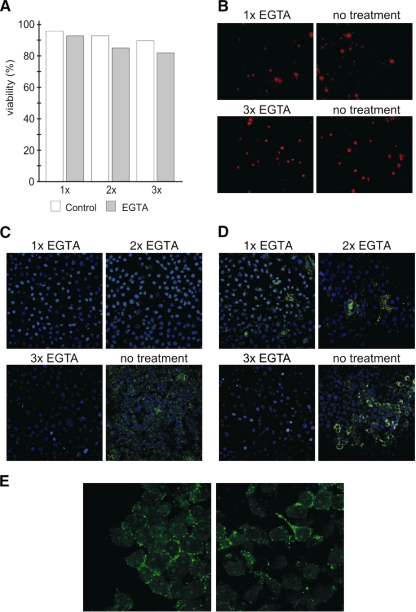
FIG. 5.
Effect of keratin network disassembly and loss of cell-cell contacts on MX LCMV spread in HeLa cells. Immunofluorescence analysis of HeLa cells mixed with HeLa-MX cells in a 10:1 ratio was used to detect MX LCMV spread. (A and B) The mixed cell monolayers were treated with EGTA, and the viability of cells was assessed by flow cytometric analysis (A) and by fluorescence microscopy of cells stained with propidium iodide (B) as described in Materials and Methods. (C and D) For analysis of MX LCMV spread, the cells were stained with desmosome (C)- and NP (D)-specific antibodies. EGTA was added to cells and left for 15 min once, twice, or three times, with 24-h recovery periods between the treatments. Untreated cells were maintained in parallel. In the absence of EGTA treatment, clusters of neighboring cells show the presence of desmosomes and NP, suggesting that the virus was spread via cell-cell contacts. In contrast, virtually no desmosomes are visible in EGTA-treated cells, and the NP signal is either confined to few isolated cells or completely absent from the monolayer. Original magnification, ×10. (E) In order to show that LCMV spread is affected by disruption of intercellular contacts (as a consequence of disruption of the keratin network), we stained nonfixed cells with NP-specific antibody. Because antibody cannot cross the membranes of living cells, it could bind only to NP present on the cell surface as a component of minimal infection units. The figure shows that NP can be detected on the cell surface and that the signal is concentrated in the areas of intercellular contacts. On the other hand, it is not visible when cell-cell contacts are missing.