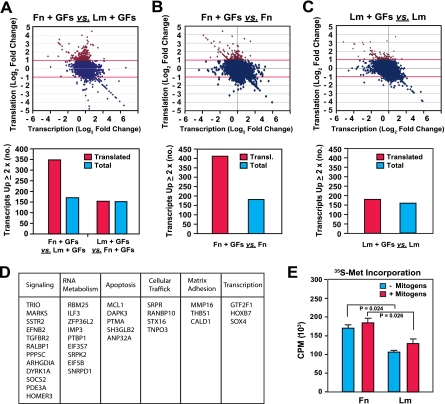
FIG. 2.
Integrin-specific signaling promotes the translation of a subset of cellular mRNAs. (A to C) Integrin-specific adhesion controls mRNA translation in cells exposed to growth factors. Total and polysome-bound mRNAs from HUVECs plated onto fibronectin (Fn) or laminin 1 (Lm) for 16 h in the absence or presence of growth factors (GFs) as described in the legend to Fig. 1B were hybridized to Affymetrix human genome U133A oligonucleotide microarray chips. Panel A shows the comparison between cells plated onto fibronectin and those plated onto laminin 1 in the presence of growth factors, panel B shows the comparison between cells plated onto fibronectin in the presence and absence of growth factors, and panel C shows the comparison between cells plated onto laminin 1 in the presence and absence of growth factors. (Top panels) The dot matrix graphs plot log2 changes in translation levels against the corresponding log2 changes in transcription levels for all genes represented on the array. Data for genes upregulated at the translational level by twofold or more are denoted in red. (Bottom panels) The bar graphs illustrate the total numbers of genes regulated at the translational (transl.) and transcriptional levels in the indicated comparisons. (D) The products of genes that were upregulated more than fourfold at the translational level in cells on fibronectin in the presence of growth factors compared to those in cells on laminin 1 under the same conditions were assigned to functional categories. (E) Integrin-specific signaling promotes protein biosynthesis. HUVECs were synchronized in G0, detached, and plated onto fibronectin or laminin for 6 h in SFM supplemented (+) or not (−) with growth factors and for 1 h in methionine-cysteine-free medium supplemented or not with growth factors (Gibco BRL). They were then labeled for 1 h with 200 μCi/ml [35S]methionine-cysteine (Tran35S-Label; ICN). The graph shows the mean levels ± SD of radioactivity present in identical aliquots of total lysates from the indicated samples.