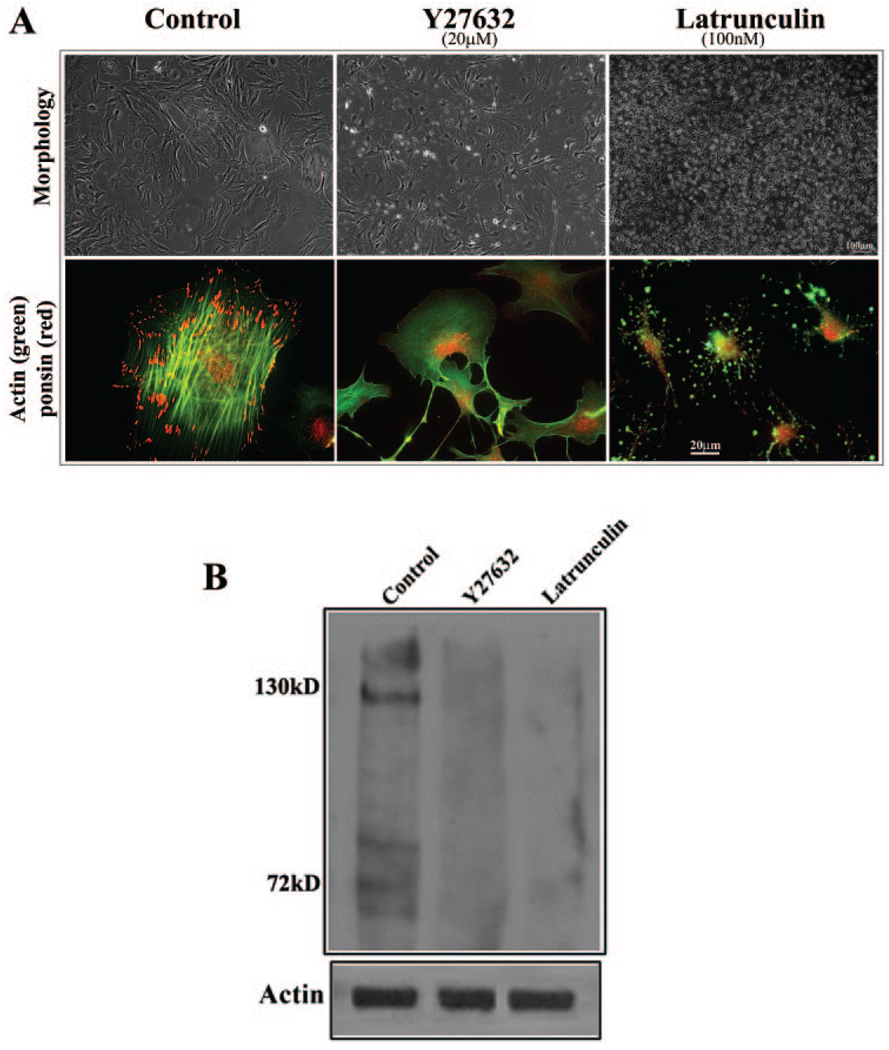
FIGURE 6.
Influence of actin polymerization on the distribution and expression of ponsin in lens epithelial cells. The potential relationship between actin filament organization and ponsin distribution and expression was explored by treating mouse lens primary epithelial cells with the Rho kinase inhibitor Y-27632 (20 µM) or latrunculin B (100 nM) and examining them for changes in ponsin distribution and levels. (A) Lens epithelial cells treated with Y-27632 and latrunculin B for 2 hours exhibited cell– cell separation and detachment and changes in cell morphology. As expected, the cells exhibited an almost complete loss of actin stress fibers (FITC fluorescence). But intriguingly, the loss of actin stress fibers was associated with a striking decrease in ponsin (red fluorescence) localization to the focal adhesions. (B) Since actin depolymerization appears to lead to a decrease in ponsin levels, ponsin levels were also evaluated by immunoblot analysis of Rho kinase inhibitor– and latrunculin B–treated (24 hours) cells. Membrane-rich insoluble fractions derived from these cells revealed a substantial reduction in ponsin protein level compared with untreated control cells. These data are representative of two independent experiments.