Table 14.
Vinylation using 20 and 23 in NaOH–H2O
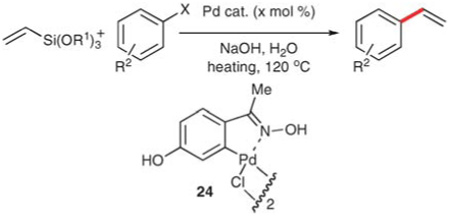 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | R1 | R2 | X | Pd cat (mol%) |
Heat source |
t/min | Yield (%) |
| 1 | Me | 4-MeC(O) | Br | Pd(OAc)2 (0.5) | µW | 10 | 97 |
| 2 | Et | 4-MeC(O) | Br | 24 (0.5) | µW | 10 | 90 |
| 3 | Me | 4-MeC(O) | Br | 24 (0.1)a | µW | 10 | 99 |
| 4 | Me | 4-MeO | I | 24 (0.1) | µW | 10 | 93 |
| 5 | Me | 4-MeO | I | 24 (0.01)a | Δ | 240 | 89 |
| 6 | Me | 3,5-(MeO)2 | I | Pd(OAc)2 (0.1)a | µW | 15 | 83 |
| 7 | Me | 4-MeO | Br | 24 (1)a | µW | 10 | 97 |
| 8 | Me | b | Br | 24 (1)a | µW | 20 | 92 |
| 9 | Me | 4-Cl | Br | 24 (1)a | µW | 15 | 71 |
| 10 | Me | c | Br | Pd(OAc)2 (0.5)d | Δ | e | 89 |
| 11 | Me | c | Br | Pd(OAc)2 (0.5)a | µW | 10 | 89 |
| 12 | Me | f | Br | 24 (1)a | µW | 15 | 97 |
| 13 | Me | 4-MeC(O) | Cl | 24 (2)a | µW | 25 | 71 |
| 14 | Me | 4-PhC(O) | Cl | 24 (2)a | µW | 25 | 65 |
TBAB (25 mol%) added.
1-Bromonaphthalene.
2-Bromo-6-methoxynaphthalene.
TBAB (200 mol%).
1 day.
3-Bromopyridine.
