Table 8.
Vinylation using 1-methyl-1-vinysiletane (15)
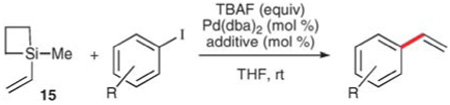 | ||||||
|---|---|---|---|---|---|---|
| Entry | R | 15 (equiv.) |
TBAF (equiv.) |
Pd(dba)2 (mol%) |
t/h | Yield (%) |
| 1 | 4-COOEt | 1.2 | 2.0 | 1 | 1 | 93 |
| 2 | 4-C(O)Me | 1.2 | 2.0 | 1 | 1 | 85 |
| 3 | 4-C(O)Mea | 1.2 | 3.0 | 2.5b | 0.5c | 75 |
| 4 | 4-NO2 | 1.2 | 2.0 | 1 | 1 | 90 |
| 5 | 4-CN | 1.2 | 2.0 | 1 | 1 | 87 |
| 6 | 4-OMe | 1.5 | 4.5 | 5d | 4 | 74 |
| 7 | 3-NO2 | 1.2 | 2.0 | 1 | 1 | 92 |
| 8 | 3-COOEt | 1.2 | 2.0 | 3 | 1 | 90 |
| 9 | 3-CH2OH | 1.2 | 2.0 | 5d | 7.5 | 79 |
| 10 | 2-NO2 | 1.2 | 2.0 | 1 | 1.5 | 86 |
| 11 | 2-COOEt | 1.2 | 3.0 | 5d | 14 | 85 |
| 12 | 2-Me | 1.2 | 3.0 | 5d | 16 | 70 |
| 13 | 2-OMe | 1.5 | 4.5 | 5d | 10 | 75 |
| 14 | e | 1.2 | 3.0 | 5 | 4 | 76 |
4-Bromoacetophenone used as substrate.
[allylPdCl]2 (2.5 mol%) used as catalyst.
40 °C.
AsPh3 (10 mol%) added to the reaction.
1-Iodonaphthalene used as substrate.
