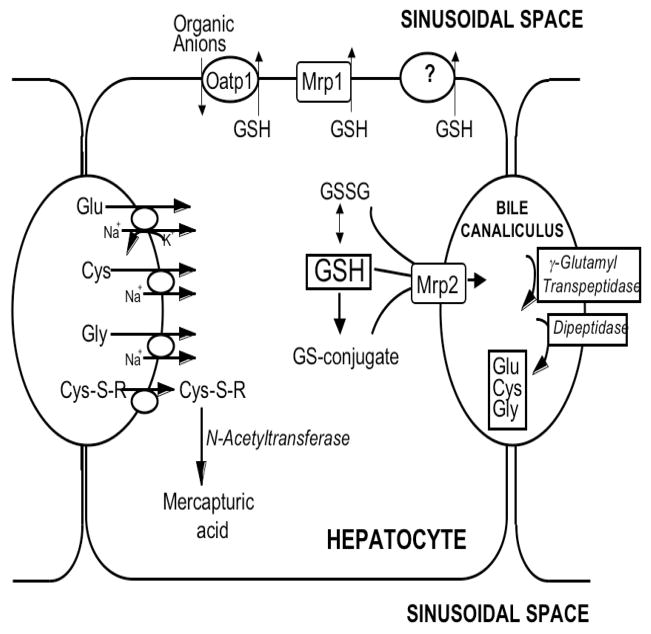
Figure 1. Glutathione homeostasis in hepatocytes.
GSH is synthesized in the cell cytosol from its precursor amino acids, glutamate, cysteine and glycine. Within the cell, it exists mainly (>98%) in the thiol-reduced form (GSH), but some is also present in the thiol-oxidized (GSSG) and as glutathione S-conjugates. After its synthesis, some of the GSH is delivered into specific intracellular compartments, including mitochondria and endoplasmic reticulum, but much of the GSH is delivered to extracellular spaces, namely blood and bile. Transport of GSH and its conjugates into bile is mediated largely by Mrp2, whereas Mrp1 and Oatp1 may contribute to GSH efflux into blood, although this is still poorly defined. In contrast to GSH synthesis, which occurs intracellularly, GSH degradation occurs exclusively in the extracellular space, and only on the surface of cells that express the ectoenzyme gamma-glutamyl transpeptidase (γGT). In the liver this enzyme is most abundant on the canalicular membrane of hepatocytes and on the apical membrane of bile duct cells. Once GSH and GSH-containing compounds are released from liver cells there is an efficient intrahepatic cycle of glutathione degradation and utilization consisting of: (a) extensive catabolism within biliary spaces, as well as within sinusoidal compartments of some species; (b) direct hepatic reabsorption of some of the breakdown products; and (c) intracellular utilization of the amino acids, or conversion of cysteine S-conjugates to mercapturic acids, i.e., N-acetylcysteine S-conjugates.