Abstract
The metabolic cost of force production, and therefore the demand for oxygen, increases with intensity and frequency of contraction. This study investigated the interaction between fatigue and oxygenation, reflected by deoxymyoglobin (dMb), during slow and rapid rhythmic isometric contractions having the same duty cycles and relative force-time integrals (FTI). We used 1H magnetic resonance spectroscopy and measures of dorsiflexor muscle force to compare dMb and fatigue (fall of maximal voluntary force, MVC) in 11 healthy adults (29+7 years, mean+SD) during 16 min of slow (4s contract/6s relax) and rapid (1.2s/1.8s) incremental (10-80% MVC) contractions. We tested the hypotheses that 1) the rate of Mb desaturation would be faster in rapid compared to slow contractions, and 2) fatigue, Mb desaturation and the fall in FTI would be greater, and PO2 (oxygen tension) lower, at the end of rapid compared to slow contractions. Although dMb increased more quickly during rapid contractions (p=0.05), it reached a plateau at a similar level in both protocols (∼42% max, p=0.49), likely due to an inability to further increase force production and thus metabolic demand. Despite the similar dMb at the end of both protocols, fatigue was greater in rapid (56.6±2.7 % baseline) compared to slow (69.5±4.0%, p=0.01) contractions. These results indicate that human skeletal muscle fatigue during incremental isometric contractions is in part a function of contraction frequency, possibly due to metabolic inhibition of the contractile process.
Keywords: myoglobin, PO2, magnetic resonance, dorsiflexors, strength, desaturation
Introduction
Skeletal muscle fatigue, defined as a drop in force-generating capacity in response to contractions, will occur as a result of changes in the biochemical milieu within the cell (Fitts 1994; Kent-Braun et al. 2002; Nosek et al. 1987). For example, intracellular acidosis and the accumulation of diprotonated inorganic phosphate, H2PO4-, have been implicated in force inhibition, both in vitro (Nosek et al. 1987; Debold et al. 2004; Knuth et al. 2006) and in vivo (Kent-Braun 1999; Degroot et al. 1993; Kent-Braun et al. 2002; Wilson et al. 1988). During incremental contractions, the intramuscular metabolic response has been shown to shift from primarily oxidative metabolism during the early, steady-state portion of exercise to a greater reliance on glycolytic energy production, with accompanying metabolite accumulation and fatigue, at higher intensities (Kent-Braun et al. 1993).
As contraction intensity increases, so will the metabolic demand. During muscular work, oxygen delivery increases to meet the increased need for oxygen by the mitochondria (Andersen and Saltin 1985; Radegran and Saltin 1998). With intense muscle contractions, the rate of oxygen consumption by the mitochondria may exceed the delivery of oxygen to the myocyte, resulting in a transient decline in intracellular oxygenation level. This gradient in oxygen concentration between the vascular bed and the mitochondria serves an important purpose by facilitating additional oxygen delivery (Wittenberg and Wittenberg 2003). However, it is unclear whether the decline in intracellular oxygenation during incremental isometric contractions also reflects an oxygen limitation that may trigger the development of muscle fatigue, possibly by causing an increase in the by-products of glycolytic metabolism.
In addition to contraction intensity, the frequency of contraction is an important determinant of the metabolic cost of muscular work. In human and animal muscle that more ATP is required to generate force than to maintain it (Russ et al. 2002; Hogan et al. 1998). Even when the force-time integral (FTI) is held constant, the metabolic demand will be higher during rapid contractions because the total number of contractions, and thus the overall requirement for force generation, is greater (Bergstrom and Hultman 1988; Hogan et al. 1998). Whether the higher metabolic demand of rapid contractions results in a greater decrease in intracellular oxygenation, and how this may relate to muscle fatigue, has not been studied.
A drop in intracellular oxygenation will be reflected by a decrease in myoglobin (Mb) saturation, as Mb unloads oxygen in an attempt to meet the oxygen demands of the mitochondria. With certain assumptions, Mb desaturation can be used to calculate intracellular oxygen partial pressure (PO2) (Richardson et al. 1995; Wang et al. 1990), which largely determines the oxygen gradient from capillary to mitochondria and therefore governs oxygen diffusion into the cell. Because the deoxygenated form of Mb (deoxymyoglobin, dMb) is visible with proton magnetic resonance spectroscopy (1H MRS) (Wang et al. 1990), Mb desaturation can be quantified continuously and non-invasively during muscle contractions, thereby providing a measure of intracellular oxygenation in vivo.
The purpose of this study was to compare Mb desaturation and fatigue during two incremental isometric contraction protocols, one with a slow contraction/relaxation cycle (“slow rhythmic”) and one with a rapid contraction/relaxation cycle (“rapid rhythmic”). We hypothesized that 1) the rate of Mb desaturation would be greater in rapid compared to slow contractions, and 2) fatigue, Mb desaturation and the fall in the force-time integral (FTI) would be greater (and PO2 lower) at the end of rapid compared to slow contractions, due to the greater metabolic demand of the rapid task.
Materials and methods
Subjects
Eleven healthy, non-smoking volunteers (6M, 5W) between the ages of 21 and 35 were studied. Subjects were free from disease and not taking any medications that might impact muscle function or blood flow. All were relatively sedentary, as determined by telephone interview. Prior to their enrollment, all subjects signed an informed consent document, approved by the University of Massachusetts Human Subjects Review Committee and the Human Investigation Committee at Yale University, and in accordance with the Declaration of Helsinki. Two visits were required, the first at the University of Massachusetts for habituation and the second at Yale University for the fatigue and MRS studies.
Habituation
Subjects reported to the laboratory following a minimum 4-hour fast. Subjects were also asked to refrain from caffeine consumption for a minimum of 4 hours, and from exercise for a minimum of 12 hours prior to all testing. After written informed consent and a health history were completed, height and mass were measured. Resting supine ankle and brachial blood pressures were measured (Wigmore et al. 2004; Wigmore et al. 2006), to ensure that the ankle/brachial systolic pressure ratio was >1.0, indicating healthy peripheral vasculature (McDermott et al. 2001). Subjects were positioned supine with a blood pressure cuff around the thigh and connected to a rapid cuff inflator and air source (D.E. Hokanson, Bellevue WA). Subjects underwent 10 min of cuff-induced ischemia (220 mmHg) to familiarize them with the ischemic procedure to be used during the fatigue studies.
Ten minutes following the bout of ischemia, subjects were positioned with the right foot in a custom-built exercise apparatus designed for isometric ankle dorsiflexion. Straps were used across the foot and leg to minimize movement. The apparatus included a force transducer (model SSM-AJ-250, Interface, Scottsdale, AZ) and computer interface, used to digitize and store the force signal (Labview Software, version 5.1, National Instruments, Austin, TX). Subjects then performed 2 isometric maximal voluntary contractions (MVC, 3-4 s each) of the ankle dorsiflexors, each separated by 2 min of rest. To ensure that all subjects could fully activate the dorsiflexor muscles, a central activation measurement was performed during a third MVC. Briefly, a supramaximal stimulus (0.1 ms pulse, 50 Hz, 250 ms train duration) was applied via percutaneous stimulation of the peroneal nerve during the MVC. The central activation ratio (CAR) was calculated as the ratio of MVC force (prior to the stimulus train) to the total force produced during stimulation. A CAR value of 1.0 represents complete voluntary muscle activation (Kent-Braun and Le Blanc 1996). Next, subjects practiced the first 3 min of the slow rhythmic contraction protocol. The subjects then were repositioned with the left foot in the exercise apparatus, and the MVCs, muscle activation measures and practice of the rapid rhythmic protocol were performed.
Fatigue Studies
Two to 14 days following the habituation visit, subjects were transported to the Magnetic Resonance Research Center at Yale University for the fatigue and dMb studies. The foot was positioned in the non-magnetic exercise apparatus, and a circular surface coil (6.5 cm diameter) tuned to the 1H frequency (170.41 MHz) was secured over the tibialis anterior muscle before positioning this muscle in the isocenter of a 4T whole-body superconducting magnet (Bruker Instruments, Billerica, MA). Gradient-echo scout images were acquired to ensure correct positioning of the tibialis anterior in the magnet and to select a region for localized shimming (FASTMAP technique, (Shen et al. 1997)). Proton spectra were acquired using a 500 μs, frequency-selective Gaussian pulse centered on the dMb resonance frequency. To minimize baseline artifacts due to partial excitation of the water signal, CHESS water suppression was applied (500 μs Gaussian pulse, centered on water, 5 ms crusher gradient).
While in the magnet, subjects performed 2 practice MVCs, separated by 2 min of rest. A third MVC was performed if the first two were more than 10% apart. The highest MVC was used to calibrate a panel of light-emitting diodes, which provided subjects with real-time force feedback. Subjects then performed the first of the two protocols. Approximately 5 min after the end of contractions, ischemia was induced in the leg for 10 min.
Proton spectra were acquired before, during and after each 16-min contraction protocol, and during the subsequent 10-min ischemic period (repetition time = 25 ms, sweep width = 30,000 Hz, number of acquisitions = 80, temporal resolution = 2 s), with 660 spectra for the contraction series (1 min rest, 16 min intermittent contractions and 5 min recovery) and 480 for the ischemic series (1 min rest, 10 min ischemia and 5 min recovery). The ischemic period was used to fully deoxygenate the muscle and provide an index of 100% dMb. We chose 10 min of ischemia, as our pilot work and the work of others (Tran et al. 1999; Wang et al. 1990) have shown that this time period is sufficient to achieve a plateau in dMb. Following recovery from ischemia, subjects were removed from the magnet and allowed ∼1.5 hour to rest before performing the second contraction protocol with the opposite leg. The order of leg and protocol (i.e., slow or rapid rhythmic) was randomized and blocked.
Contraction Protocols
Both protocols consisted of eight 2-min stages of intermittent contractions, starting at 10% MVC, and increasing by 10% every 2 min (up to a target of 80% MVC). To assess muscle fatigue, an MVC was performed at the start of each stage and after the final contraction of the last stage. To minimize the effect of the MVC on changes in dMb, a brief rest period (16 s) followed each MVC, and the force and dMb data from the first 30s of each stage were excluded from further analyses. The only difference between the two fatigue protocols was the cadence of contractions. The contraction/relaxation cycle was 4 s/6 s for the slow and 1.2 s/1.8 s for the rapid rhythmic protocols, respectively. The slow protocol was selected because it transitions from a low-intensity, oxidative portion to a more metabolically-demanding, fatiguing portion (Kent-Braun et al. 1993). Further, it was anticipated that the 4 s contraction could induce some degree of occlusion at the higher contraction intensities, thereby emphasizing the need to adequately reperfuse the muscle during the 6 s recovery period. The rapid protocol was included because it is more energetically-demanding than slow contractions and would require a stronger vasoreactive response to maintain adequate oxygen delivery. Thus, the rapid protocol was expected to produce more dMb and fatigue than the slow protocol. The duty cycle (40%), and therefore total contraction time, was the same in both protocols. Subjects were cued to contract and relax by a tone on an audio recording.
Data Analysis
Muscle Force
Force values were converted to Newtons based on linear calibration of the force transducer, and peak MVC force and the FTI during each contraction were calculated. These values were then scaled to each subject’s peak force and FTI from the baseline MVC, respectively, and averaged to give 30 s time resolution.
Deoxymyoglobin (dMb)
Using NUTS software (Acorn, Livermore, CA), dMb data were summed to yield 30-s time resolution (i.e., 15 free-induction decays). Data were zero-filled, and multiplied by a mixed Lorentzian/Gaussian function (-300 Hz, 0.1) prior to Fourier transformation. Spectra were phased (0 and 1st order) and baseline-corrected before an automated curve-fitting procedure was applied to estimate the dMb peak area for each spectrum. Accurate phasing and curve-fitting were verified. For each ischemic trial, the highest dMb peak area was identified and a 3-point average including that point was calculated and termed 100% dMb. For each leg, all other dMb peak areas obtained during contractions and ischemia were scaled to this value. Oxygen partial pressure was then calculated throughout each protocol, as follows:
where P50 is PO2 at 50% oxygen saturation, which was set to 2.5 Torr (Wittenberg and Wittenberg 2003; Wang et al. 1990).
The rate of increase in dMb between minutes 4 and 12 of contractions was investigated for both protocols in each individual using linear regression analysis. This time range was chosen because 1) dMb did not rise appreciably until after min 4, and 2) beyond minute 12, FTI was no longer matched in the two protocols. Thus, this time range represented a phase of similar, incrementing contraction intensities in the slow and rapid protocols during which changes in dMb were readily detectable.
Statistical Analyses
Baseline MVC (N) was compared across legs using paired t-tests. The time of fatigue onset (i.e., decrease in MVC from baseline) in each protocol was determined by repeated measures ANOVA. End-exercise values for FTI, dMb, PO2 and fatigue (MVC, % baseline) were compared across contraction protocols using paired t-tests, as were the slopes from the individual linear fits of dMb and time. Mean, SD and precise p-values are given in tables; the figure shows mean and SE. Significance was considered established when p ≤ 0.05.
Results
Isometric strength (MVC, N) was greater in the right compared to left leg (p = 0.05, Table 1). Mean CAR was > 0.98 in both legs, indicating the ability of the subjects to achieve essentially full activation of the unfatigued dorsiflexor muscles.
Table 1.
Subject characteristics
| Age (years) | 29 (7) |
| Height (cm) | 165.1 (25.2) |
| Mass (kg) | 75.2 (33.2) |
| Right Leg MVC (N) | 361.2 (99.6) |
| Left Leg MVC (N) | 334.3 (106.2) |
| Right Leg CAR | 1.00 (0.01) |
| Left Leg CAR | 0.98 (0.03) |
Note: Data are mean (SD). n = 11 except for CAR, where n = 10.
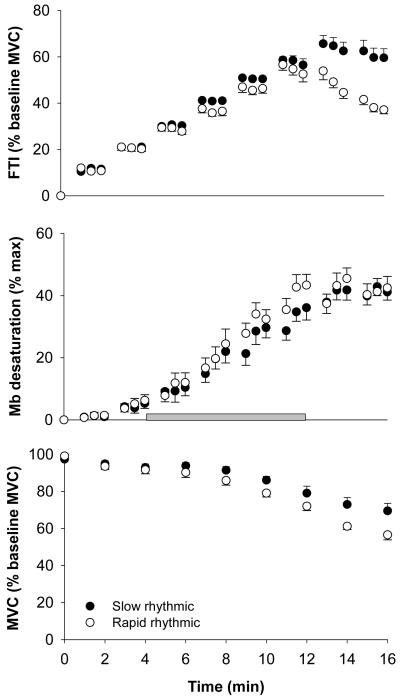
Figure 1 shows the changes in FTI (top) and MVC (bottom) during 16 min of slow (solid) and rapid (open) rhythmic contractions. The onset of fatigue occurred earlier during the rapid compared to the slow protocol (min 4 vs. min 10, respectively, p = 0.05). At the end of the protocols (min 16), FTI (p < 0.001) and MVC (p = 0.01) were lower in the rapid compared to the slow protocol, indicating greater fatigue during rapid rhythmic contractions (Table 2).
Figure 1. Changes in FTI (top), dMb (middle) and MVC (bottom) for slow and rapid contractions.
FTI (top) changed similarly during the early stages of both protocols. However, the onset of fatigue (fall of MVC from baseline, bottom) occurred earlier in the rapid contraction protocol (min 4) than in the slow protocol (min 10). Subjects fatigued more by the end of the rapid protocol (min 16), as demonstrated by a greater fall in FTI and MVC at the end of rapid compared with the slow contractions (p < 0.001 for FTI and p = 0.01 for MVC). Although the rate of increase in dMb (min 4-12, shaded bar) was greater during rapid compared to slow contractions (p = 0.05), there was no difference in dMb between protocols at the end of the contraction period. Data are mean + SE.
Table 2. Force and oxygenation variables at the end of each protocol.
| FTI (% baseline) | MVC (% baseline) | dMb (% maximal) | PO2 (Torr) | |
|---|---|---|---|---|
| Slow Rhythmic Contractions | 59.6 (11.7) n = 9 |
69.5 (13.4) | 41.2 (8.6) | 3.82 (1.37) |
| Rapid Rhythmic Contractions | 37.2 (5.1) n = 9 |
56.6 (8.9) | 42.5 (11.7) n = 10 |
3.84 (1.93) n = 10 |
| p-value | < 0.001 | 0.01 | 0.49 | 0.89 |
Note: FTI (force-time integral), MVC (maximal voluntary contraction force), dMb (deoxymyoglobin) and PO2 (oxygen partial pressure) are provided (mean (SD); p-values are for the difference between protocols at min 16; n = 11 except where indicated.
The greater fatigue during rapid contractions was accompanied by a faster increase in dMb (Figure 1, middle, shaded bar), reflected by the steeper slope of dMb versus time for rapid (0.085 + 0.024 %·min-1, mean ± SD) compared with slow (0.066 ± 0.020 %·min-1) contractions (p = 0.05). This occurred despite the similar FTI in the two protocols between minutes 4 and 12 (Figure 1, top). By the end of contractions, dMb and PO2 were similar between protocols (Table 2).
Discussion
In the human ankle dorsiflexor muscles, we observed greater fatigue following rapid compared to slow rhythmic isometric contractions of the same duty cycle. This difference in fatigue was accompanied by a more rapid rise in Mb desaturation during the rapid rhythmic protocol, which may reflect the higher oxygen transport required by the mitochondria to meet the greater metabolic cost of higher-frequency contractions (Hogan et al. 1998). Despite the differences in fatigue at the end of the protocols, dMb and PO2 were similar and well above the purported critical PO2 of ∼0.5 Torr. Thus, our first hypothesis was supported, but our second hypothesis was not. These results provide new insight about the interactions between oxygen state and fatigue during incremental contractions of varying frequency in human skeletal muscle in vivo.
During the slow contraction protocol, subjects were able to attain target force through ∼60% MVC, which is consistent with the performance of this protocol in previous studies (Kent-Braun et al. 2002; Wigmore et al. 2006). However, it is apparent that the rapid protocol was more taxing, resulting in a decline in force production (FTI) and greater fatigue (fall of MVC) during the latter stages of this protocol compared to the slow rhythmic protocol (Figure 1). The greater fatigue during rapid contractions was accompanied by a steeper increase in dMb compared to slow contractions. The fatigue and dMb data are both consistent with the concept that muscle contractions at high frequencies are more metabolically costly (i.e., require more ATP) than those at low frequencies (Ferguson et al. 2001). During isometric contractions, this higher metabolic demand has been attributed to the greater metabolic cost of force generation compared to that of force maintenance (Russ et al. 2002; Bergstrom and Hultman 1988). Although the duty cycle, and therefore the total contraction time, was the same in both protocols, more contractions were generated in the rapid rhythmic protocol compared to the slow rhythmic protocol (20 contractions/min and 6 contractions/min, respectively). Thus, we had expected that the greater energy demand of the rapid contraction protocol would be accompanied by greater Mb desaturation, due to an increased rate of oxidative phosphorylation and oxygen consumption by the mitochondria.
Another factor that may have contributed to the varying dMb responses during rapid and slow contractions is oxygen delivery. It is well established that blood flow increases with increasing contraction intensity up to the point of full vascular occlusion (Wigmore et al. 2004; Barnes 1980; Barcroft and Millen 1939; Lind and Williams 1979). Although the contraction intensity that induces full occlusion is unresolved and may depend on the muscle group involved, our previous work using functional magnetic resonance imaging indicates that full occlusion occurs at ∼60% MVC in the ankle dorsiflexor muscles (Wigmore et al. 2004). While the contraction intensities achieved during the latter stages of the contraction protocols used in the current study would most likely be sufficient to induce some degree of occlusion, particularly in the slow rhythmic protocol, the intermittent nature of the protocol provided an opportunity for oxygen delivery between contractions. It is likely that the slow rhythmic contractions induced more vascular occlusion, and hence lower oxygen delivery, than did the rapid contractions. However, it is apparent that this did not have a large impact on net muscle oxygenation, as dMb rose more quickly during rapid contractions. Further, any transient reduction in blood flow during the 4 s contractions was unlikely to affect force production, as force production is not limited by blood flow during the slow rhythmic contraction protocol (Wigmore et al. 2006).
In skeletal muscle, Mb is thought to act as a spatial buffer for oxygen, shuttling oxygen from the cytosol to the mitochondria (Wittenberg and Wittenberg 2003). When mitochondrial demand increases, Mb provides an immediate source of oxygen to the mitochondria, resulting in an initial decline in Mb saturation and cellular PO2. Oxygen delivery then increases in response to the O2 gradient that develops between the vasculature and the mitochondria (Wittenberg and Wittenberg 2003). As observed here and by others (Mole et al. 1999; Richardson et al. 1995; Richardson et al. 2001), Mb desaturates progressively during incremental contractions as metabolic demand increases, at least through 50-60% of maximal exercise intensity. The increase in dMb, and therefore the drop in intracellular PO2, that accompanies each increment in contraction intensity increases the oxygen gradient from blood to cytosol, further facilitating oxygen diffusion into the cell.
During the incremental contractions used here, force production became limited before whole-muscle critical PO2 was reached. Although dMb rose more sharply in the rapid protocol, dMb at the end of the contractions was similar in both protocols. The most likely explanation for the similar end-exercise dMb is that, during the latter stages of the protocols, FTI reached a plateau (slow protocol) or declined (rapid protocol), thereby curtailing the metabolic demand for oxygen and the need for further perturbations to the intracellular oxygen state. The intracellular PO2 at the end of the two protocols was well above the suggested values for critical PO2 (Connett et al. 1990; Gayeski et al. 1987), below which muscular performance becomes impaired. This result is consistent with the relatively modest degree of fatigue developed during these protocols.
Myoglobin desaturation reached a plateau in both contraction protocols, despite progressive muscle fatigue and a shift from submaximal to essentially maximal contractions. The response of dMb to contractions of increasing intensity has been the focus of several earlier studies. Richardson and colleagues found Mb desaturation to peak at ∼50% of maximal at ∼50-60% of maximal work rate, and this dMb level was maintained as exercise intensity was increased to maximal (1995). Conversely, Mole et al. (1999) reported that dMb increased linearly with whole body VO2, ultimately reaching ∼ 48% desaturation. In the current study, dMb increased with contraction intensity to a plateau that was accompanied by a plateau (slow) or a decline (rapid) in force production (Figure 1). While it is possible that Mb would have desaturated further had the subjects been able to continue to increase the workload, we cannot address this question without additional study. Likewise, it is possible that the PO2 in some cells was very low, and it was these cells that were failing to produce force. As our measure of desaturation captures the average dMb in the tissue sample and not the desaturation level of individual cells, we cannot make that determination in the current study.
An advantage of 1H MRS measures of dMb is that it provides information on intracellular oxygen, rather than on a combination of dMb + deoxy-hemoglobin, such as obtained with near-infrared spectroscopy (NIRS). Despite this difference, a number of comparisons are useful here. Mizuno et al. (2004) recently observed that, during incremental isometric contractions in the knee extensor muscles, an increase in the rate of oxygen desaturation was observed at ∼30-40% of MVC, which agrees reasonably well with our data (Figure 1). In contrast, during whole-body cycling exercise in highly-trained athletes, an increased rate of desaturation was not observed until ∼62% of peak oxygen consumption (Grassi et al. 1999). Finally, Hicks et al. (1999) reported an ∼10% fall in oxygenation within the first 15 s of an isometric forearm contraction held at 30% MVC, while observing no significant change in oxygenation by NIRS for a contraction at 10% MVC. At the same time, however, measures of venous oxygen saturation did detect a decrease in oxygenation during the 10% contraction, prompting the authors to urge some caution in interpreting NIRS data. Overall, however, the NIRS data are consistent with the notion of an intensity-dependent fall in tissue oxygenation, as observed in our study.
In earlier work using the slow rhythmic protocol, both blood flow (measured by post-contraction hyperemia) and H2PO4- (determined by 31phosphorus MRS) increased with contraction intensity, and H2PO4-, but not blood flow, was related to the development of muscle fatigue (Kent-Braun et al. 2002; Wigmore et al. 2006). We found blood flow to be tightly coupled to force production (i.e., FTI), such that they increased and plateaued in parallel. From the pattern of Mb desaturation (Figure 1) and the calculated changes in PO2 in the present study, we observed that intracellular oxygenation also changes in parallel with FTI. In contrast to the responses of blood flow and PO2, H2PO4- increases throughout the contraction protocol, despite the plateau in force. Further, changes in [H2PO4-] were strongly associated with the fall of MVC (Kent-Braun et al. 2002), suggesting a direct role for [H2PO4-] in the development of fatigue. Thus, in the present study, it is reasonable to infer that the accumulation of force-inhibiting metabolites, such as H2PO4-, may have played a role in the development of fatigue in these protocols.
A potential limitation of this study is the lack of data assessing the possibility of central fatigue during the incremental contraction protocols. Based on earlier work in similar subjects (Kent-Braun et al. 2002), little central activation failure (end-exercise CAR = 0.97-0.98; similar fall of MVC and electrically-evoked force) would be expected in the slow rhythmic protocol. We do not have data about central fatigue for the rapid protocol, as the brevity of these contractions make it unsuitable for the CAR measure. Thus, we cannot eliminate the possibility that some central fatigue may have occured in the rapid protocol. Were this the case, it could have contributed to the lack of further change in dMb at the end of this protocol, as there may have been some recovery of intracellular oxygenation in those fibers that were no longer active.
In summary, rapid contractions (20 per min) in the dorsiflexor muscles of healthy young adults resulted in a more rapid increase in dMb and greater fatigue than slow contractions (6 per min) performed with the same duty cycle and target forces. Fatigue at the end of the protocols appears to be independent of end-exercise intracellular oxygen content, as reflected by dMb. Rather, the disparity in fatigue may be related to differences in the metabolic demand and consequent metabolite accumulation between the two protocols.
Acknowledgements
The authors thank Douglas Rothman, Ph.D., John Buonaccorsi, Ph.D., Mike Tevald, P.T., Ph.D., Peter Brown, Mark Abildgaard, Linda Chung, M.S., Damien Callahan, M.S. and Steve Foulis for their contributions to this work. Thank you to the volunteers who participated in this study. This work is dedicated to the memory of John S. Leigh, Jr, who pioneered the use of proton MRS for the study of deoxymyoglobin in vivo. Support was from NIH R01AG21094, NIH K02AG023582, American College of Sports Medicine NASA Space Physiology Research Grant and American Heart Association Pre-doctoral Fellowship.
Contributor Information
Danielle M. Wigmore, Department of Kinesiology, University of Massachusetts, Amherst MA, dwigmor1@fsc.edu.
Douglas E. Befroy, Department of Internal Medicine—Endocrinology, Yale University School of Medicine, New Haven CT, douglas.befroy@yale.edu
Ian R. Lanza, Department of Kinesiology, University of Massachusetts, Amherst MA, lanza.ian@mayo.edu.
Jane A. Kent-Braun, Department of Kinesiology, University of Massachusetts, Amherst MA, janekb@kin.umass.edu
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J. Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. The relationship between maximum isometric strength and intramuscular circulatory occlusion. Ergonomics. 1980;23(4):351–357. doi: 10.1080/00140138008924748. [DOI] [PubMed] [Google Scholar]
- Barcroft H, Millen JLE. The blood flow through muscle during sustained contraction. J. Physiol. 1939;612:741–761. doi: 10.1113/jphysiol.1939.sp003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom M, Hultman E. Energy cost and fatigue during intermittent electrical stimulation of human skeletal muscle. J. Appl. Physiol. 1988;65:1500–1505. doi: 10.1152/jappl.1988.65.4.1500. [DOI] [PubMed] [Google Scholar]
- Connett RJ, Honig CR, Gayeski TE, Brooks GA. Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. J. Appl. Physiol. 1990;68:833–842. doi: 10.1152/jappl.1990.68.3.833. [DOI] [PubMed] [Google Scholar]
- Debold EP, Dave H, Fitts RH. Fiber type and temperature dependence of inorganic phosphate: implications for fatigue. Am. J. Physiol. 2004;287:C673–C681. doi: 10.1152/ajpcell.00044.2004. [DOI] [PubMed] [Google Scholar]
- Debold EP, Romatowski J, Fitts RH. The depressive effect of Pi on the force-pCa relationship in skinned single muscle fibers is temperature dependent. Am. J. Physiol. 2006;290:C1041–C1050. doi: 10.1152/ajpcell.00342.2005. [DOI] [PubMed] [Google Scholar]
- Degroot M, Massie BM, Boska M, Gober J, Miller RG, Weiner MW. Dissociation of [H+] from fatigue in human muscle detected by high time resolution 31P-NMR. Muscle and Nerve. 1993;16:91–98. doi: 10.1002/mus.880160115. [DOI] [PubMed] [Google Scholar]
- Ferguson RA, Ball D, Krustrup P, Aagaard P, Kjaer M, Sargeant AJ, et al. Muscle oxygen uptake and energy turnover during dynamic exercise at different contraction frequencies in humans. J. Physiol. 2001;536:261–271. doi: 10.1111/j.1469-7793.2001.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994;74(1):49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Grassi B, Quaresima V, Marconi C, Ferrari M, Cerretelli P. Blood lactate accumulation and muscle deoxygenation during incremental exercise. J. Appl. Physiol. 1999;87(1):348–355. doi: 10.1152/jappl.1999.87.1.348. [DOI] [PubMed] [Google Scholar]
- Gayeski TEJ, Connett RJ, Honig CR. Minimum intracellular Po2 for maximum cytochrome turnover in red muscle in situ. Am. J. Physiol. 1987;252(5):H906–H915. doi: 10.1152/ajpheart.1987.252.5.H906. [DOI] [PubMed] [Google Scholar]
- Hicks A, McGill S, Hughson RL. Tissue oxygenation by near-infrared spectroscopy and muscle blood flow during isometric contractions of the forearm. Can. J. Appl. Physiol. 1999;24(3):216–230. doi: 10.1139/h99-018. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Ingham E, Kurdak SS. Contraction duration affects metabolic energy cost and fatigue in skeletal muscle. Am. J. Physiol. 1998;274:E397–E402. doi: 10.1152/ajpendo.1998.274.3.E397. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur. J. Appl. Physiol. Occup. Physiol. 1999;80(1):57–63. doi: 10.1007/s004210050558. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle and Nerve. 1996;19(7):861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Miller RG, Weiner MW. Phases of metabolism during progressive exercise to fatigue in human skeletal muscle. J. Appl. Physiol. 1993;75(2):573–580. doi: 10.1152/jappl.1993.75.2.573. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J. Appl. Physiol. 2002;93(5):1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- Knuth S, Dave H, Peters JR, Fitts RH. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30 and 15 degrees centigrade: Implications for muscle fatigue. J. Physiol. 2006;575:887–899. doi: 10.1113/jphysiol.2006.106732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind AR, Williams CA. The control of blood flow through human forearm muscles following brief isometric contractions. J. Physiol. 1979;288:529–549. [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286(13):1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Tokizawa K, Iwakawa T, Muraoka I. Inflection points of cardiovascular responses and oxygenation are correlated in the distal but not the proximal portions of muscle during incremental exercise. J. Appl. Physiol. 2004;97(3):867–873. doi: 10.1152/japplphysiol.00213.2004. [DOI] [PubMed] [Google Scholar]
- Mole PA, Chung YR, Tran TK, Sailasuta N, Hurd R, Jue T. Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am. J. Physiol. 1999;277(1):R173–R180. doi: 10.1152/ajpregu.1999.277.1.R173. [DOI] [PubMed] [Google Scholar]
- Nosek TM, Fender KY, Godt RE. It is diprotonated inorganic-phosphate that depresses force in skinned skeletal-muscle fibers. Science. 1987;236(4798):191–193. doi: 10.1126/science.3563496. [DOI] [PubMed] [Google Scholar]
- Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J. Physiol. 1995;486(Pt 3):689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am. J. Physiol. 1998;43(1):H314–H322. doi: 10.1152/ajpheart.1998.274.1.H314. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Newcomer SC, Noyszewski EA. Skeletal muscle intracellular Po-2 assessed by myoglobin desaturation: response to graded exercise. J. Appl. Physiol. 2001;91(6):2679–2685. doi: 10.1152/jappl.2001.91.6.2679. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J. Clin. Invest. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW, Elliott MA, Vandenborne K, Walter GA, Binder-Macleod SA. Metabolic costs of isometric force generation and maintenance of human skeletal muscle. Am. J. Physiol. 2002;282:E448–E457. doi: 10.1152/ajpendo.00285.2001. [DOI] [PubMed] [Google Scholar]
- Shen J, Rycyna RE, Rothman DL. Improvements on an in vivo automatic shimming method (FASTERMAP) Magn. Reson. Med. 1997;38(5):834–839. doi: 10.1002/mrm.1910380521. [DOI] [PubMed] [Google Scholar]
- Tran TK, Sailasuta N, Kreutzer U, Hurd R, Chung YR, Mole P, et al. Comparative analysis of NMR and NIRS measurements of intracellular PO2 in human skeletal muscle. Am. J. Physiol. 1999;276(6):R1682–R1690. doi: 10.1152/ajpregu.1999.276.6.R1682. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Noyszewski EA, Leigh JS. In vivo MRS measurement of deoxymyoglobin in human forearms. Magn. Reson. Med. 1990;14(3):562–567. doi: 10.1002/mrm.1910140314. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lannergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J. Physiol. 1997;500(Pt 1):193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore DM, Damon BM, Pober DM, Kent-Braun JA. MRI measures of perfusion-related changes in human skeletal muscle during progressive contractions. J. Appl. Physiol. 2004;97(6):2385–2394. doi: 10.1152/japplphysiol.01390.2003. [DOI] [PubMed] [Google Scholar]
- Wigmore DM, Propert K, Kent-Braun JA. Blood flow does not limit skeletal muscle force production during incremental isometric contractions. Eur. J. Appl. Physiol. 2006;96(4):370–378. doi: 10.1007/s00421-005-0037-0. [DOI] [PubMed] [Google Scholar]
- Wilson JR, McCully KK, Mancini DM, Boden B, Chance B. Relationship of muscular fatigue to pH and diprotonated Pi in humans: a 31P-NMR study. J. Appl. Physiol. 1988;64:2333–2339. doi: 10.1152/jappl.1988.64.6.2333. [DOI] [PubMed] [Google Scholar]
- Wittenberg JB, Wittenberg BA. Myoglobin function reassessed. J. Exp. Biol. 2003;206(12):2011–2020. doi: 10.1242/jeb.00243. [DOI] [PubMed] [Google Scholar]