Abstract
Despite recognized evidence for the importance of the multifidus muscle in stabilizing the lumbar spine, identifying subjects at risk for injury and subsequent loss of intrinsic spinal stabilization remains difficult. Previous research has failed to associate multifidus muscle size and height, weight, or body mass index (BMI). The purpose of this study was to begin to establish normative data for the multifidus muscle cross-sectional area (CSA) at the L5 level and to identify factors associated with size. Twenty-five participants (17 female), with a mean age of 32.5 (SD 11.6) years without history of LBP were considered for inclusion. Participants' height and weight were recorded and BMI calculated. Ultrasound imaging was used to obtain a CSA in cm2 of the subjects' multifidus muscles at the L5 level bilaterally; testing was done by two trained testers. Prior to testing, intra- and inter-tester reliability were determined. Percent body fat was determined using a three-site skinfold caliper measurement, also using two trained testers. Mean BMI was 24.18 and mean body fat (%) was 22.88 for all participants. As expected, age and BMI were moderately correlated. Left and right multifidus muscle CSA were highly correlated (r = 0.92, p < 0.001). The mixed model ANOVA indicated a significant main effect for gender as males exhibited larger CSA than females. Participants without history of low back pain present with symmetrical multifidus muscle CSA at the L5 level. Clear gender differences in CSA show that males tend to have larger multifidus muscles at the L5 level, indicating a need to establish gender-specific norms for clinicians examining the L5 multifidus muscle.
KEYWORDS: Lumbar Spine, Multifidus, Sonography
Perhaps one of the most frequent health complaints in modern medicine, low back pain (LBP) remains a burden to individuals and society. The prevalence of LBP and its associated disability, including the related financial burden, remain ever-increasing in the U.S.1 and among other industrialized countries2. Each year in the U.S., for example, it is estimated that approximately 15% of adults will experience an episode of LBP lasting two weeks or longer3. The World Health Organization estimates the cost of work-related LBP at 818,000 lost disability-adjusted life years annually4. LBP as a condition is induced by a variety of wide-ranging factors, which may be categorized into two classes in the development of the disorder: internal or endogenous (genotypical and phenotypical factors) and external or exogenous (physical and psychosocial)5.
While identification of participants at risk for injury and subsequent loss of intrinsic spinal stabilization remains difficult, and a plethora of risk factors have been identified as interacting6, there is recognized evidence for the importance of the multifidus muscle in stabilizing the lumbar spine7–10. Previous research has established fat-free mass as the best correlate to maximal back strength, regardless of gender in a pain-free population (males: r = 0.67; females: r = 0.64)11. In addition, the multifidus muscle cross-sectional area, gender, and pain on exertion are powerful predictors of back muscle strength in participants with chronic LBP12,13. However, prior research has failed to conclusively associate measures of nutritional status (e.g., body mass index (BMI), the relation between body weight and the square of height) and back muscle strength14,15 or the incidence of LBP16.
It is well established that patients with chronic LBP present with a weakness impairment of the lumbar multifidus muscles17. Gross lower body weakness is also associated with LBP, in particular, weakness of the hip flexors and adductors and, the transversus abdominis muscles, but loss of muscle power in the back extensor muscles, including the multifidus, predominates18,19. This weakness, as evidenced by multifidus muscle atrophy that can be seen clearly on ultrasound imaging, is injury-side and level specific20.
The multifidus is a deep back muscle occupying the groove between the transverse and spinous processes and is best developed in the lumbar region21. Here it is comprised of fascicles of varying lengths arranged in a spino-transverse pattern. Innervation of each fascicle of a given lumbar level (and the facet joint of that level) is derived from the medial branch of the dorsal ramus22. Aspden23 has suggested that this segmental innervation allows the multifidus the ability to control or adjust a particular segment to match applied loads. In regards to size, the cross-sectional area of the multifidi gradually increase on progression from L2-S124.
The purpose of this study was to begin to establish normative data of the multifidus muscle cross-sectional area (CSA) measured bilaterally at the L5 level through ultrasound imaging and to identify factors associated with size. Strength of muscle is related to CSA of muscle. In both male and female groups25–27 as well as old and young28, there exists a positive correlation between muscle strength and cross-sectional area. Those factors that correlate with smaller multifidus muscle CSA might be implicated as potential risk factors for lumbar injury. Prior to this study, a reliability study was conducted to establish the tester's ability to accurately detect muscle size.
Methods
Participants
Twenty-five student, faculty, and staff member volunteers (17 females; mean age 32.5 +/– 11.6) were recruited from the Department of Physical Therapy at Western Carolina University. Consecutive participants (age range 23–62) without history of LBP were considered for inclusion. Participants had a mean body weight of 155 lbs (range 112 to 225 lbs) and height of 67 in (range 61 to 75 in). Patients with scoliosis, previous back surgery, or arthritis were excluded. The study was approved by the Institutional Review Board of Western Carolina University for investigation with human participants. Informed written consent was obtained from each participant prior to investigation.
Materials and Procedures
Participants' height and weight were recorded and BMI calculated. Percent body fat was determined using a three-site skinfold measurement with a Jamar caliper (Preston, Jackson, MI). Measurements were taken on the right side of the body by two experienced observers. For male participants, chest, abdominal, and thigh locations were recorded; and for female participants the triceps, suprailium, and thigh locations were recorded.
Using a 5.0–3.5 MHz convex-array transducer (sono AQUILA, Biosound, Indianapolis, IN), ultrasound imaging was used by two trained testers to obtain the CSA, in cm2, of the participants' multifidus muscles at the L5 level bilaterally. A standardized protocol was used as previously established by Hides et al29,30 that placed the participants in prone with 35° of hip flexion and no lumbar lordosis. The L5 multifidus muscle has been identified as being the richest in muscle spindle density24 and having the largest CSA30,31. Thus, the L5 spinal segment was chosen instead of the S1 segment used by previous investigators30 because it appears to be the best indicator of dynamic lumbar stability. The L5 spinal segment was identified according to protocol by palpating the posterior superior iliac spines and marking with ink the spinous process between the two, regarded as the S1 spinous process. Testers then palpated superiorly and marked the location of the L5 spinous processes for reference. Copious gel was applied across the marked location. Hyperechoic margins marked the lamina and spinous process margins visually, verifying the inferior and medial boundaries of the multifidus muscle, respectively. Each participant was asked to perform a contralateral extension straight leg raise, creating an isolated ipsilateral multifidus contraction in order to confirm the muscle location before imaging in the resting position. Care was taken not to compress the tissue with the transducer while imaging. When the tester was satisfied with the multifidus representation, the image was frozen and the elliptical tool was selected. The CSA of the multifidus was measured by tracing with electronic calipers around the margin of the muscle at the time of imaging (direct tracing) by marking the superior margin (subcutaneous fascial plane), inferior margin (lamina), medial margin (against spinous process), and lateral margin (longissimus fascial plane). A sample multifidus image is shown in Figure 1. The process was then repeated on the opposite side.
FIGURE 1.
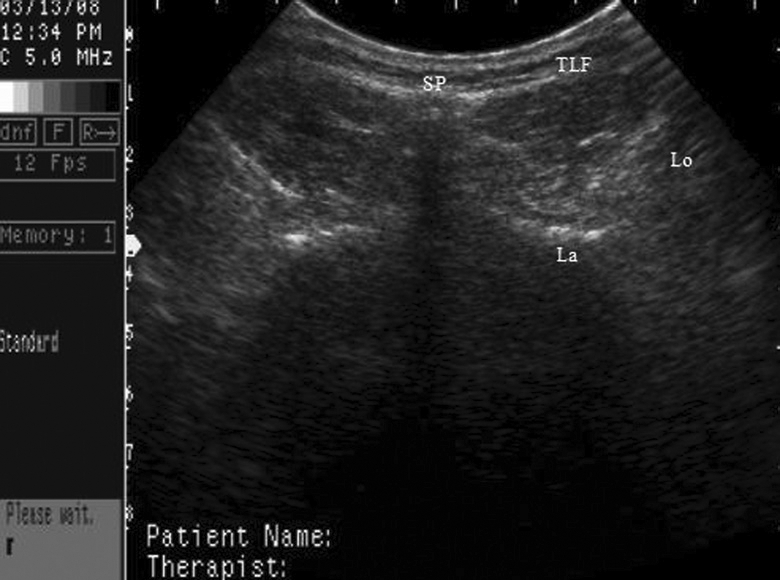
Rehabilitation ultrasound imaging of L5 multifidus muscle CSA. This multifidus CSA image is of a female. Boundaries for measurement are the spinous process (SP), lamina (La), thoracolumbar fascia (TLF), and longisimuss (Lo) muscle border.
Reliability
To establish reliability, measures were taken on two different days, according to the previously described method by Martinson and Stokes32 for training physical therapists in measurement of muscle CSA. Prior to participant testing in the multifidus study, intra- and inter-tester reliability were established on two trained testers. Originally six testers were trained extensively on multifidus CSA. Following training, each tester performed measurements of six individuals' CSA of the tibialis anterior muscle (right leg) as per the Martinson and Stokes method. Testers also tested these same individuals one day later. Two out of the six testers were randomly selected to perform the multifidus CSA study.
Statistics
Intraclass correlations (ICC3,1) were used to examine reliability among testers. Inter-tester reliability was examined via the second-day data collection; an ANOVA was used to examine absolute differences among testers as well. Intra-tester reliability for each tester was examined during the first- and second-day data collections. Pearson correlations were conducted to examine relationships between age and BMI, percent body fat and BMI, and percent body fat and multifidus CSA. A 2 (gender) × 2 (left and right multifidus) mixed model ANOVA with repeated measures on last factor was conducted to examine gender differences on CSA scores. A one-way between-subjects ANCOVA was calculated to examine the effect of gender on L5 multifidus size, covarying out the effect of percent body fat.
Data were screened for assumptions of statistical tests where appropriate. Significance for all statistical tests was set at .05; effect sizes and observed power were reported when appropriate. The SPSS software (version 15.0) was used for data analysis.
Results
For the reliability testing prior to the study, intra-tester reliability obtained on days one and two indicated ICC > 0.95 for all testers. Inter-tester reliability obtained on the second test day indicated ICC = 0.92; absolute reliability among testers indicated no significant differences between testers (p > .05). Thus, these findings indicated high reliability for testing protocols among these testers.
Descriptive data for each participant on L5 multifidus muscle CSA and anthropometric measurements are presented in Table 1. Mean descriptive data for males, females, and total subjects regarding age, height, weight, and nutritional status measures (mean BMI and percent body fat) are reported for all participants by gender in Table 2. Also included in Table 2 are left and right multifidus muscle CSA group values.
TABLE 1.
Descriptive data from 25 participants without history of LBP.
| Subject (n = 25) | Age (yr) | Gender | Height (in) | Weight (lb) | BMI | Left Multif. (cm2) | Right Multif. (cm2) | Body Fat (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 24 | F | 65 | 125 | 20.80 | 5.53 | 5.28 | 23.00 |
| 2 | 23 | F | 67 | 124 | 19.40 | 6.03 | 5.65 | 16.30 |
| 3 | 33 | M | 72 | 170 | 23.10 | 8.01 | 7.48 | 14.20 |
| 4 | 25 | M | 69 | 170 | 25.10 | 8.06 | 8.67 | 5.30 |
| 5 | 24 | F | 61 | 120 | 22.70 | 7.16 | 6.03 | 27.80 |
| 6 | 26 | M | 75 | 225 | 29.50 | 8.80 | 8.58 | 22.80 |
| 7 | 41 | M | 68 | 170 | 25.80 | 10.68 | 9.61 | 14.40 |
| 8 | 23 | F | 65 | 140 | 23.30 | 5.87 | 6.60 | 30.40 |
| 9 | 29 | F | 62 | 117 | 21.40 | 8.17 | 7.96 | 20.00 |
| 10 | 25 | F | 69 | 152 | 22.40 | 6.03 | 5.65 | 20.80 |
| 11 | 26 | F | 69 | 142 | 21.00 | 7.04 | 7.04 | 21.90 |
| 12 | 49 | F | 67 | 205 | 32.10 | 5.87 | 5.53 | 35.40 |
| 13 | 41 | M | 72 | 185 | 25.10 | 5.28 | 5.53 | 22.20 |
| 14 | 26 | F | 62 | 145 | 26.50 | 5.34 | 5.31 | 36.70 |
| 15 | 33 | M | 70 | 180 | 25.80 | 7.48 | 7.54 | 23.40 |
| 16 | 27 | F | 66 | 130 | 21.00 | 5.65 | 6.60 | 25.00 |
| 17 | 40 | F | 65 | 150 | 24.60 | 5.53 | 5.65 | 23.20 |
| 18 | 62 | F | 67 | 221 | 34.60 | 6.03 | 6.41 | 41.10 |
| 19 | 57 | F | 68 | 180 | 27.40 | 6.41 | 5.53 | 31.00 |
| 20 | 25 | M | 67 | 148 | 23.20 | 6.60 | 6.53 | 19.00 |
| 21 | 23 | F | 66 | 123 | 19.90 | 5.87 | 5.72 | 18.60 |
| 22 | 23 | F | 63 | 150 | 26.60 | 5.65 | 6.03 | 25.00 |
| 23 | 31 | M | 71 | 150 | 20.90 | 6.03 | 6.41 | 7.20 |
| 24 | 52 | F | 61 | 112 | 20.80 | 5.18 | 5.28 | 24.20 |
| 25 | 24 | F | 67 | 138 | 21.60 | 5.53 | 5.28 | 23.00 |
Multif. = multifidus muscle at the L5 segment
TABLE 2.
Descriptive data, nutritional status, and multifidus muscle CSA by gender.
| Age (Yr) | Height (in) | Weight (lb) | BMI | %BF | CSA Left | CSA Right | |
|---|---|---|---|---|---|---|---|
| Male | 31.88 (6.53) | 70.50 (2.56) | 175.75 (24.06) | 24.81 (2.53) | 16.06 (7.03) | 7.59 (1.67) | 7.57 (1.38) |
| Female | 32.76 (13.55) | 65.29 (2.64) | 145.53 (30.44) | 23.88 (4.30) | 26.08 (6.79) | 6.05 (0.76) | 5.97 (0.73) |
| Total | 32.48 (11.62) | 66.96 (3.56) | 154.88 (31.31) | 24.18 (3.80) | 22.88 (8.24) | 6.54 (1.32) | 6.48 (1.39) |
Values are means and (SD). The averaged (left and right) L5 multifidus muscle CSA for male subjects (mean = 7.58 cm2, SD = 1.51) was larger (p = 0.002) than that of female subjects (mean = 6.01 cm2, SD = 0.70). No differences were noted for left and right multifidus muscle CSA (p = 0.67). %BF = percent body fat. CSA = cross-sectional area of multifidus muscle at the L5 level.
As expected, age and BMI were moderately correlated (r = .59, p = 0.002). Left (mean = 6.54, SD = 1.32) and right (mean = 6.48, SD = 1.39) multifidus muscle CSA were highly correlated (r = 0.92, p < 0.001). Interestingly, moderately strong inverse relations were noted for percent body fat and multifidus CSA (r = −0.44 for right and −0.41 for left, p < 0.05). The mixed model ANOVA indicated a significant main effect for gender (F1,23 = 12.8, p = 0.002, η2 = 0.36, observed power = 0.929) as males (mean = 7.58, SD = 1.51) exhibited larger CSAs than females (mean = 6.01, SD = 0.70). Also, this effect was moderate even though there were fewer male than female participants. The main effect for left and right multifidus muscle was not significant (F1,23 = 0.19, p = 0.67, η2 = 0.008, observed power = 0.058) (mean = 6.54, SD = 1.32; mean = 6.48 SD = 1.23, respectively). Additionally, the interaction was not significant (F1,23 = 0.19, p = 0.71, η2 = 0.003) since gender differences were consistent (parallel) for left and right multifidus muscles (see Table 2) as males (mean = 7.57, SD = 1.38 for right; mean = 7.59, SD = 1.67 for left) had larger multifidus muscle CSAs than females (mean = 5.97, SD = 0.73 for right; mean = 6.05, SD = 0.76 for left). To ensure that gender differences in CSA were a function of maleness or femaleness and not simply a function of differences of percent body fat that may be expressed in males and females, an ANCOVA was used. The average L5 multifidus CSA was significantly related to gender (F1,23 = 6.30, p = 0.02). The main effect for percent body fat was not significant (F1,23 = 0.41, p > 0.05), with males having significantly larger multifidus CSAs than females even after covarying out the effect of percent body fat.
Discussion
The multifidus muscle is the most medial of the lumbar back muscles and substantially covers the lumbosacral area33. Due to the ovoid shape of multifidus muscle, a convex array transducer provides more information than a linear transducer and is easily distinguished on all sides except the lateral, where it adjoins with longissimus34. Since multifidus muscle is deep and quite difficult to palpate or manually muscle test, ultrasound is an ideal assessment tool for examination of this muscle30.
Given that our findings indicate a very high degree of L5 multifidus side-to-side symmetry in healthy subjects, identification of CSA asymmetry in patients with LBP should be considered evidence for involvement at that level, as has been shown previously by Hides et al29, who established high concordance between MRI imaging versus ultrasound in examining the L2 through S1 multifidus muscle in 10 asymptomatic females. The average CSA was the largest at L5 (7.12cm2), then S1 (6.44cm2), followed by L4 (4.87cm2). They found that all their healthy subjects had a side-to-side percent difference < 5% while the LBP subjects differed by > 8%. While our male subjects were < 5% (mean = 4.97), our females were measured above this marker (mean = 6.45) but still below the 8% difference seen in LBP subjects. Further investigation of multifidus atrophy in LBP subjects has been shown to decrease on the painful side, as well as at the clinically identified level of symptom provocation35.
While Pressler et al30 found the average CSA for left S1 multifidus muscle (4.18, SD 0.55 cm2) to be slightly larger than the right (4.11, SD 0.57 cm2) in a group of 30 healthy females, the Pearson's correlation (r = 0.94, p < 0.05) between right and left CSA was high and a t-test indicated no difference in side-to-side CSA symmetry. Our group of 17 females likewise were found to have side-to-side symmetry with an average CSA for left L5 multifidus muscle of 6.05 cm2 (SD 1.28), which was larger than the right multifidus muscle CSA average of 5.97 cm2 (SD 1.15). Our correlation between right and left CSA was equally high (r = 0.91, p < .05), and our t-test also indicated no difference in side-to-side CSA symmetry (p > .05).
Additionally, our current findings indicate a need to establish gender-specific multifidus muscle CSA reference norms, as males were significantly larger at L5. The findings of our female mean that multifidus CSA and coefficient of variance are consistent with previous findings in asymptomatic subjects. Our data pool of female subjects (N = 17) had an average multifidus CSA of 6.01 cm2 and coefficient of variation (CV%) value of 11.7. Hides, Richardson, and Jull29 (N=10) found that asymptomatic female subjects' CSA and CV percentages were 6.19 cm2 and 4.02 respectively, while Stokes, Rankin, and Newham34 found that their female subjects (N=46) had multifidus CSAs of 6.65 cm2 and 15.0.
Previously, Hides, Cooper, and Stokes36 examined height, weight, and BMI as possible correlates with L4 multifidus muscle CSA. Males were found to have significantly larger CSA than females (6.15 cm2 vs 5.60 cm2). They found no correlation between BMI and multifidus muscle CSA in either males or females.
Stokes, Rankin, and Newham34 also found that male multifidus muscle CSA was greater when compared to that of females, prior to being normalized by body mass, with no significant differences in symmetry between genders. Age differences were only found in shape, not size. Multifidus muscle was found to be bigger at the L5 vertebral segment when compared to L4. The authors concluded that neither height, weight, nor BMI attributed to significant differences in multifidus muscle CSA. This was thought to occur because the multifidus muscle is not a weight-bearing muscle; therefore, a relationship of its size to weight or height would not be expected.
Other gender differences noted included our finding that while male and female subjects had very similar BMI (24.81 vs. 23.88), there was a significant difference (p < .01) in percent body fat (16.06 vs. 26.08). These findings are similar to a previous imaging study37 that indicated that females had a higher proportion of body fat even though their BMI was similar to that of the male subjects.
Hicks et al38 examined trunk muscle area and muscle attenuation (higher fatty infiltrate) in healthy men (N=739) and women (N=788) aged 70–79 using computed tomography. Posterior trunk, lateral trunk wall, and rectus abdominis muscle were imaged using axial CT scans at the L4–L5 disc space, and an average score was calculated for both muscle area (multifidus and erector spinae muscles together for the posterior trunk) and attenuation. Using a standardized performance measure for estimating functional capacity, they found no association with muscle area (p > 0.10). However, high fatty infiltrate was positively associated with poorer performance as well as with a history of LBP.
What is unusual and unexpected in our findings is the moderately strong inverse relationship found between percent total body fat and the L5 multifidus muscle CSA. This correlation may implicate increasing percent body fat as a potential risk factor for loss of lumbar stability. Visser et al39 examined the relations of skeletal muscle mass and percent body fat with self-reported physical disability in 753 elderly men and women (ages 72 to 95) using cross-sectional data from the biennial examination of the Framingham Heart Study. Body composition was measured via dual-energy x-ray absorptiometry (DEXA), and muscle mass was reported as either total body or lower extremity. They found that body composition was strongly associated with physical disability in old age. As expected, high percent body fat was associated with an increased estimated risk of mobility disability. Muscle mass (both total body and lower extremity) was not associated with disability in either men or women, although the multifidus itself was not measured. Additionally, it should be noted that total body skeletal muscle was estimated from the fat-free and bone-free mass assessed by DEXA. This finding is likely valid in the lower extremity; however, validity is threatened for total body muscle mass as the total body estimate included the viscera and organs. Body composition changes with age are typified by increases in percent body fat and decreases in lean mass and muscle mass40,41. As a progressively sedentary lifestyle leads to increases in body fat deposition and infiltration, there is likely some degree of co-morbid disuse atrophy of the lumbar multifidus muscle at the L5 level. As the deep laminar fibers of the multifidus are essential for segmental joint protection and stability, their atrophy would facilitate loss of the dynamic stabilizing capacity of the lumbar spine. While this sedentary effect has not been demonstrated directly among non-LBP subjects, several studies have demonstrated multifidus morphological changes after onset of LBP. Therefore, while the findings of a moderately inverse relationship between the L5 multifidus muscle CSA and percent total body fat is mere conjecture based upon our 25 subjects, it bears future consideration in larger data pools that seek to establish reference norms.
Limitations
Certainly, multifidus muscle size may vary day to day due to hydration or other factors. Performing a within-day as well as between-day reliability study prior to the collection of data would have helped rule out error from these factors in the study design.
We chose to focus our study on the L5 multifidus because it has been demonstrated to be the largest among the L2-S1 muscles, and it has the greatest potential for providing dynamic stability to its segment. It has been reported that the borders of the L2 to L5 multifidus are more irregular and difficult to visualize. For this reason, Pressler et al30 focused on the S1 multifidus due to its ease of visualization for using the sonographic ellipse tool for CSA measurement. This irregularity may have affected measurement accuracy.
Additionally, no conclusions may be drawn from the noted inverse relation of percent body fat and multifidus size. As we were attempting to establish normative data on healthy subjects, no information regarding physical activity level was recorded.
Finally, while our subject sample does have diversity of anthropometrics and age, future studies should seek a larger sample size that is similarly diverse and one that includes a greater proportion of males.
Conclusions
The findings of our present study corroborate previous investigations of the lumbar multifidus, namely in participants without history of LBP, where there is a high degree of side-to-side symmetry at the L5 level, and where males have larger CSAs than females at that level. These findings support the need to establish gender-specific norms for clinicians to reference when examining a patient's L5 multifidus.
Acknowledgements
We would like to thank Candice Wells, PT, and Cameron Coghill, PT, for their assistance in data collection on this project.
REFERENCES
- 1.Frymoyer JW. Predicting disability from low back pain. Clin Orthop Relat Res. 1992;279:101–109. [PubMed] [Google Scholar]
- 2.Spitzer WO, Leblanc FE, Dupuis M. Scientific approach to the assessment and management of activity-related spinal disorders. Report of the Quebec Task Force on Spinal Disorders. Spine. 1987;12:516–520. [PubMed] [Google Scholar]
- 3.Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Punnett L, Pruss-Utun A, Nelson DI, et al. Estimating the global burden of low back pain attributable to combined occupational exposures. Am J Ind Med. 2005;48:459–469. doi: 10.1002/ajim.20232. [DOI] [PubMed] [Google Scholar]
- 5.Celan D, Turk Z. The impact of anthropomorphic parameters on the incidence of low back pain. Coll Antropol. 2005;1:101–105. [PubMed] [Google Scholar]
- 6.Sprince N, Park H, Zwerling C, et al. Risk factors for low back injury among farmers in Iowa: A case-control study nested in the agricultural health study. J Occup Environ Hyg. 2007;4:10–16. doi: 10.1080/15459620601067266. [DOI] [PubMed] [Google Scholar]
- 7.Gedalia U, Solomonow M, Zhou BH, Baratta RV, Lu Y, Harris M. Biomechanics of increased exposure to lumbar injury caused by cyclic loading. Part 2. Recovery of reflexive muscular stability with rest. Spine. 1999;24:2461–2467. doi: 10.1097/00007632-199912010-00007. [DOI] [PubMed] [Google Scholar]
- 8.Solomonow M, Zhou B, Baratta RV, Lu Y, Zhu M, Harris M. Biexponential recovery model of lumbar viscoelastic laxity and reflexive muscular activity after prolonged cyclic loading. Clin Biomech. 2000;15:167–175. doi: 10.1016/s0268-0033(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 9.Solomonow M, Zhou BH, Baratta RV, Lu Y, Harris M. Biomechanics of increased exposure to lumbar injury caused by cyclic loading: Part 1. Loss of reflexive muscular stabilization. Spine. 1999;24:2426–2434. doi: 10.1097/00007632-199912010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Solomonow M, Zhou BH, Harris M, Lu Y, Baratta RV. The ligamento-muscular stabilizing system of the spine. Spine. 1998;23:2552–2562. doi: 10.1097/00007632-199812010-00010. [DOI] [PubMed] [Google Scholar]
- 11.Mannion AF, Kaser L, Weber E, Rhyner A, Dvorak J, Muntener M. Influence of age and duration of symptoms on fibre type distribution and size of the back muscles in chronic low back pain patients. Eur Spine J. 2000;9:273–281. doi: 10.1007/s005860000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, De Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9:266–272. doi: 10.1007/s005860000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller A, Johansen JG, Hellesnes J, Brox JI. Predictors of isokinetic back muscle strength in patients with low back pain. Spine. 1999;24:275–280. doi: 10.1097/00007632-199902010-00016. [DOI] [PubMed] [Google Scholar]
- 14.Oddsson L, Giphart JE, Buijs R, Roy SH, Taylor HP, DeLuca CJ. Development of new protocols and analysis procedures for the assessment of LBP by surface EMG techniques. J Rehabil Res Dev. 1997;34:415–426. [PubMed] [Google Scholar]
- 15.Larivière C, Gravel D, Gagnon D, Arsenault AB, Loisel P, Lepage Y. Back strength cannot be predicted accurately from anthropometric measures in subjects with and without chronic low back pain. Clin Biomech. 2003;18:473–479. doi: 10.1016/s0268-0033(03)00026-3. [DOI] [PubMed] [Google Scholar]
- 16.Pollack KM, Sorock GS, Lade MD, et al. Association between body mass index and acute traumatic workplace injury in hourly manufacturing employees. Am J Epidemiol. 2007 doi: 10.1093/aje/kwm058. ([Epub ahead of print]). [DOI] [PubMed] [Google Scholar]
- 17.Danneels LA, Coorevits PL, Cools AM, et al. Differences in electromyographic activity in the multifidus muscle and the iliocostalis lumborum between healthy subjects and patients with sub-acute and chronic low back pain. Eur Spine J. 2002;11:13–19. doi: 10.1007/s005860100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newcomer KL, Jacobson TD, Gabriel DA, Larson DR, Brey RH, An KN. Muscle activation patterns in subjects with and without low back pain. Arch Phys Med Rehabil. 2002;83:816–821. doi: 10.1053/apmr.2002.32826. [DOI] [PubMed] [Google Scholar]
- 19.Nourbakhsh MR, Arab AM. Relationship between mechanical factors and incidence of low back pain. J Orthop Sports Phys Ther. 2002;32:447–460. doi: 10.2519/jospt.2002.32.9.447. [DOI] [PubMed] [Google Scholar]
- 20.Hides J, Gilmore C, Stanton W, Bohlscheid E. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man Ther. 2008;13:43–49. doi: 10.1016/j.math.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Moore KL, Dalley AF. Clinically Oriented Anatomy. Philadelphia: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 22.Bogduk N. Clinical Anatomy of the Lumbar Spine and Sacrum. Edinburgh, UK: Elsevier; 2005. [Google Scholar]
- 23.Aspden RM. Review of the functional anatomy of the spinal ligaments and the lumbar erector spinae muscles. Clin Anat. 1992;5:372–387. [Google Scholar]
- 24.Amonoo-Kuofi HS. The density of muscle spindles in the medial, intermediate and lateral columns of human intrinsic postvertebral muscles. J Anat. 1983;136:509–519. [PMC free article] [PubMed] [Google Scholar]
- 25.Maughan RJ, Watson JS, Weir J. Strength and cross-sectional area of human skeletal muscle. J Physiol. 1983;338:37–49. doi: 10.1113/jphysiol.1983.sp014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maughan RJ, Watson JS, Weir J. Relationships between muscle strength and muscle cross-sectional area in male sprinters and endurance runners. Eur J Appl Physiol Occup Physiol. 1983;50:309–318. doi: 10.1007/BF00423237. [DOI] [PubMed] [Google Scholar]
- 27.Maughan RJ, Watson JS, Weir J. Muscle strength and cross-sectional area in man: A comparison of strength-trained and untrained subjects. Br J Sports Med. 1984;18:149–157. doi: 10.1136/bjsm.18.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young A, Stokes M, Crowe M. Size and strength of the quadriceps muscles of old and young women. Eur J Clin Invest. 1984;14:282–287. doi: 10.1111/j.1365-2362.1984.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 29.Hides JA, Richardson CA, Jull GA. Magnetic resonance imaging and ultrasonography of the lumbar multifidus muscle: Comparison of two different modalities. Spine. 1995;20:54–58. doi: 10.1097/00007632-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Pressler JF, Heiss DG, Buford JA, Chidley JV. Between-day repeatability and symmetry of multifidus cross-sectional area measured using ultrasound imaging. J Orthop Sports Phys Ther. 2006;36:10–18. doi: 10.2519/jospt.2006.36.1.10. [DOI] [PubMed] [Google Scholar]
- 31.Rankin G, Stokes M. Reliability of assessment tools in rehabilitation: An illustration of appropriate statistical analyses. Clin Rehabil. 1998;12:187–199. doi: 10.1191/026921598672178340. [DOI] [PubMed] [Google Scholar]
- 32.Martinson H, Stokes MJ. Measurement of anterior tibial muscle size using real-time ultrasound imaging. Eur J Appl Physiol Occup Physiol. 1991;63:250–254. doi: 10.1007/BF00233856. [DOI] [PubMed] [Google Scholar]
- 33.Macintosh JE, Valencia F, Bogduk N, Munro RR. The morphology of the human lumbar multifidus. Clin Biomech (Bristol, Avon) 1986;1:196–204. doi: 10.1016/0268-0033(86)90146-4. [DOI] [PubMed] [Google Scholar]
- 34.Stokes M, Rankin G, Newham DJ. Ultrasound imaging of lumbar multifidus muscle: Normal reference ranges for measurements and practical guidance on the technique. Man Ther. 2005;10:116–126. doi: 10.1016/j.math.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. 1994;19:165–172. doi: 10.1097/00007632-199401001-00009. [DOI] [PubMed] [Google Scholar]
- 36.Hides J, Cooper DH, Stokes MJ. Diagnostic ultrasound imaging measurement of lumbar multifidus muscle in normal young adults. Physiother Theory and Pract. 1992;8:19–26. [Google Scholar]
- 37.Stokes M, Young A. Measurement of quadriceps cross-sectional area by ultrasonography: A description of the technique and its application to physiotherapy. Physiother Theory Pract. 1986;2:31–36. [Google Scholar]
- 38.Hicks GE, Simonsick EM, Harris TB, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study: The moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60:882–887. doi: 10.1093/gerona/60.7.882. [DOI] [PubMed] [Google Scholar]
- 39.Visser M, Harris TB, Langlois J, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–221. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 40.Borkan GA, Norris AH. Fat redistribution and the changing body dimensions of the adult male. Hum Biol. 1977;49:495–513. [PubMed] [Google Scholar]
- 41.Flynn MA, Nolph GB, Baker AS, Martin WM, Krause G. Total body potassium in aging humans: A longitudinal study. Am J Clin Nutr. 1989;50:713–717. doi: 10.1093/ajcn/50.4.713. [DOI] [PubMed] [Google Scholar]


