Abstract
BACKGROUND:
The role of KATP channels in isoflurane’s reducing effects on oxygen free radical formation are not well known.
OBJECTIVES:
To investigate whether glyburide, an ATP-regulated potassium (KATP) channel blocker, abolishes isoflurane-induced cardioprotective effects and whether it affects hydroxyl radical formation in the postischemic reperfused heart.
ANIMALS AND METHODS:
Thirty-nine male Wistar rats were divided into four groups: group C (control, n=10), group I (isoflurane, n=9), group G (glyburide, n=10) and group GI (glyburide and isoflurane, n=10). The hearts were perfused as a Neely’s working heart model. Afterwards, global heart ischemia was induced for 15 min followed by reperfusion for 20 min. The formation of hydroxyl radicals in the coronary effluent and heart was measured with high performance liquid chromatography.
RESULTS:
Isoflurane alone and glyburide alone produced significant decreases in the duration of ventricular fibrillation during reperfusion (group C 452±345, group I 247±60, group G 261±135 s; P<0.05). In the presence of glyburide, isoflurane did not further decrease the duration of arrhythmia (group GI 230±48 s). Isoflurane reduced hydroxyl radical formation significantly in the coronary effluent during ischemia and reperfusion, but this was prevented by glyburide.
CONCLUSION:
The results suggest that isoflurane reduces hydroxyl radical formation, at least in part, through activation of KATP channels.
Keywords: Glyburide, Hydroxyl radical, Isoflurane, KATP channels
It has been reported that inhalation anesthetics have cardioprotective effects against ischemic and reperfusion injuries (1–4). However, the mechanism has not been fully elucidated. Recently, activation of ATP-regulated potassium (KATP) channels has been reported to be one of the important mechanisms for the cardioprotective effect of isoflurane (5). KATP channel agonists enhance the functional recovery of postischemic-reperfused myocardium, decrease myocardial infarct size and mimic the effects of myocardial ischemic preconditioning in vivo. Conversely, these effects are abolished by pretreatment with KATP channel antagonist (6–8).
On the other hand, hydroxyl radicals are produced during myocardial ischemia and reperfusion. Volatile anesthetics have been reported to prevent hydroxyl radical production in the ischemic heart (9–11). The relation between the KATP channel and hydroxyl radicals is not well known. Therefore, it is interesting to investigate whether glyburide, a KATP channel blocker, abolishes the isoflurane-induced cardioprotective effects and whether it affects hydroxyl radical formation in the postischemic reperfused heart.
ANIMALS AND METHODS
This study was approved by the Animal Ethics Committee of Yamanashi Medical University. The technique was used in an earlier study (11). Briefly, 39 three-month-old male Wistar rats weighing 280 to 320 g were used. They were randomly divided into four groups as follows: group C (control, n=10), group I (isoflurane, n=9), group G (glyburide, n=10) and group GI (glyburide and isoflurane, n=10). The animals in each group were sufficiently anesthetized with 5% isoflurane for about 7 min. The hearts were then rapidly excised and perfused according to the Langendorff procedure. Nonrecirculating modified Krebs-Henseleit bicarbonate buffer was used as a preperfusate. The perfusate was maintained at 37.0±0.3°C and contained (in mM) NaCl 118, KCl 4.7, CaCl2 2.0, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, disodium-EDTA 0.5 and glucose 11. The solution was equilibrated with a gas mixture of 95% O2 and 5% CO2. In addition, 1 mM salicylic acid was added to the buffer. During Langendorff perfusion, the left atrium was connected through a pulmonary vein to an angled steel cannula. After this preliminary perfusion, the heart was converted to a working preparation for a stabilization period of 10 min. In this preparation, the perfusate was ejected from the heart into an aortic bubble trap, placed above the heart. The afterload was maintained at a constant level by setting the height of the aortic bubble trap above the level of the heart (60 mmHg).
Left ventricular pressure was measured with a transducer (P10EZ, Gould, USA) connected to a thin catheter (18 gauge, Argyle Intramedicut Catheter, Japan) inserted into the left ventricle through the mitral valve from the angled steel cannula in the left atrium. Rates of tension development (dP/dt) were measured from the derivatives of left ventricular pressure obtained electronically. Aortic outflow was recorded with an electromagnetic blood flow meter (MFV-3200, Nihon Kohden, Japan). An electrocardiogram was recorded through electrodes attached to the heart. Coronary flow was measured by timed collections of the pulmonary artery outflow and surface runoff of the heart resulting from coronary sinus and Thebesian vessel drainage. Cardiac output was considered to be the sum of the aortic and coronary outflows. At no time was the coronary effluent recirculated.
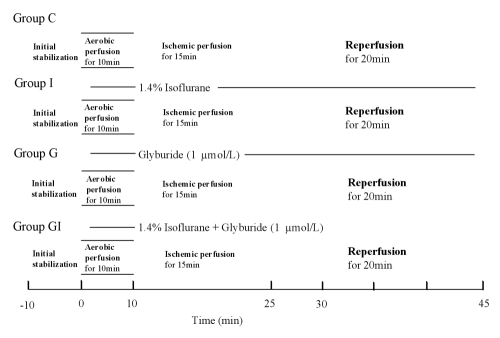
After initial stabilization (10 min), the heart was exposed for 10 min to the perfusate equilibrated with 1.4% isoflurane in the oxygenating chamber from preischemia to the end of reperfusion in groups I and GI. The concentration of anesthetic was measured continuously in the gas phase of the oxygenating chamber by an Acoma Anesthetic Agent Monitor (Acoma, Japan). In groups G and GI, the perfusate was switched to 1 μmol/L of glyburide, the KATP channel antagonist. Afterwards, whole heart ischemia was induced by clamping the one-way aortic valve bypass for 15 min (12). During the ischemic period, the heart was paced at 333 beats/min. Reperfusion of the heart after ischemia was performed by declamping the one-way aortic valve bypass tube and lasted for 20 min (Figure 1).
Figure 1).
Experimental protocol. Group C (control, n=10), group I (1.4% isoflurane, n=9), group G (glyburide 1 μmol/l, n=10), group GI (glyburide and isoflurane, n=10)
At the end of perfusion, the heart was quickly frozen in liquid nitrogen and was freeze-dried for six days. The tissue was minced into small pieces and homogenized in a polytron. An aliquot was extracted with perchloric acid and centrifuged at 3000 g. The supernatant was used for determination of dihydroxybenzoic acids (DHBAs). A small amount of perfusate was collected from the coronary effluent just before ischemia (0 min), during ischemia (just before reperfusion), and 1, 5, 10 and 20 min after reperfusion (that is, 16, 20, 25 and 35 min after the start of ischemia). The perfusate was also used for determination of DHBAs.
Determination of DHBAs
Hydroxyl radicals react with salicylic acid yielding 2,3-, 2,4-and 2,5-DHBAs. The liquid chromatography apparatus consists of a Shimazu Model LC-10AD pump and a detector module (C R4A, CHROMATOPAC, Shimazu Co Ltd, Japan). The column used was a Shim-Pack, CLC-ODS, 15 cm × 4.6 mm. The mobile phase was 90% of 20 mM sodium dihydrogen phosphate, 1 mM octanesulphonic acid sodium salt and 10 mM sodium sulphate, and 10% acetonitrile at a flow rate of 0.5 mL/min. The mobile phase was kept anaerobic by N2 (DEGASSER, DGU-3A, Shimazu Co Ltd). The detector was set at a detector voltage of 0.6 V. All samples were measured against external standards of 2,3-, 2,4- and 2,5-DHBAs and salicylic acid. One millilitre of effluent was treated with 1 ml 1 M Tris buffer and extracted with 2 ml methanol on a vortex mixer for 10 min. Alumina 50 mg was added to this and the methanol layer was separated. The residue was dissolved in 200 μl of 0.2 M perchloric acid, and 5 μl of this solution was injected into the liquid chromatography-electrochemical detection unit. These methods were modified as described by Floyd et al (13,14).
Data are expressed as mean ± SD. Analyses of significant differences in the time-dependent changes between the four groups were determined by two-way analysis of variance with repeated measures, followed by paired t tests with the Bonferroni correction. The data of DHBAs in the four groups were analyzed by one-way analysis of variance, followed by Fisher’s PLSD tests. The duration of ventricular fibrillation was analyzed by Kruskal-Wallis test. A probability of P<0.05 was regarded as statistically significant.
RESULTS
Isoflurane produced significant decreases in the duration of ventricular fibrillation during reperfusion (group C 452±345, group I 247±60 s). Glyburide also decreased the duration of fibrillation (group G 261±135 s). In the presence of glyburide, isoflurane did not show further change in the duration of arrhythmia (group GI 230±48 s).
During the preischemic period, there were no significant differences in coronary flow between the four groups. During reperfusion, coronary flow was reduced in group G compared with groups C and I except at 5 and 10 min after reperfusion. There were no significant differences in heart rate between the four groups during the whole period. Before ischemia, cardiac output was reduced in groups I and GI. The same applies to group I in maximal left ventricular dP/dt (Table 1).
TABLE 1.
Changes in heart rate, cardiac output, coronary flow and maximal rate of left ventricular tension development (LV dP/dt max)
| −10 | 0 (I) | 5 (I) | 10 (I) | 15 (I) | Time (min) 16 (R1) | 20 (R5) | 25 (R10) | 30 (R15) | 35 (R20) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Heart rate (beats/min) | ||||||||||
| Group C | 260±40 | 253±32 | 333 | 333 | 333 | 94±54‡ | 141±90‡ | 219±50‡ | 239±29 | |
| Group I | 261±47 | 250±43 | 333 | 333 | 333 | 60±35‡ | 162±68‡ | 226±40 | 227±35‡ | |
| Group G | 280±22 | 251±42 | 333 | 333 | 333 | 115±48‡ | 151±79‡ | 222±45‡ | 231±39‡ | |
| Group GI | 258±40 | 233±33 | 333 | 333 | 333 | 76±52‡ | 118±79‡ | 207±33‡ | 210±23‡ | |
| Coronary flow (mL/min) | ||||||||||
| Group C | 16.4±1.3 | 16.2±1.5 | 0.8±0.4‡ | 0.6±0.4‡ | 0.6±0.4‡ | 12.7±2.1‡ | 14.5±1.2‡ | 13.9±1.8‡ | 13.6±1.9‡ | 13.7±1.3‡ |
| Group I | 16.3±1.2 | 16.3±1.7 | 0.5±0.3*‡ | 0.5±0.3‡ | 0.3±0.3‡ | 12.7±2.4‡ | 14.5±1.4‡ | 13.3±1.7‡ | 14.4±1.5‡ | 14.5±1.5‡ |
| Group G | 18.0±1.9 | 15.7±2.3 | 0.6±0.2‡ | 0.3±0.2*‡ | 0.2±0.2*‡ | 10.2±2.7*†‡ | 16.1±1.4*† | 13.5±2.8‡ | 11.4±2.1*†‡ | 11.5±1.9*†‡ |
| Group GI | 17.9±3.0 | 15.7±2.4 | 0.5±0.1*‡ | 0.3±0.1*‡ | 0.1±0.0*‡ | 10.7±1.8‡ | 15.4±1.5 | 13.9±1.6‡ | 12.9±1.8‡ | 12.9±1.8†‡ |
| Cardiac output (mL/min) | ||||||||||
| Group C | 61.3±5.1 | 60.3±4.3 | 0.8±0.4‡ | 0.6±0.4‡ | 0.6±0.4‡ | 0.6±0.8‡ | 0.6±1.1‡ | 15.1±16.7‡ | 25.6±15.5‡ | 35.8±14.5‡ |
| Group I | 58.6±5.2 | 50.2±9.7* | 0.5±0.3‡ | 0.5±0.3‡ | 0.3±0.3‡ | 1.3±1.7‡ | 1.2±1.8‡ | 9.9±11.9‡ | 20.8±8.7‡ | 26.6±8.7*‡ |
| Group G | 62.7±3.9 | 59.5±8.0† | 0.6±0.2‡ | 0.3±0.2‡ | 0.2±0.2‡ | 1.7±1.2‡ | 3.2±5.3‡ | 9.3±9.5‡ | 21.7±5.4‡ | 30.3±6.5‡ |
| Group GI | 61.5±8.0 | 52.1±7.7*§ | 0.5±0.1‡ | 0.3±0.1‡ | 0.1±0.0‡ | 1.7±2.2‡ | 1.2±1.9‡ | 5.3±6.8‡ | 16.0±5.8*‡ | 22.1±6.2*‡ |
| LV dP/dt max (mmHg/s) | ||||||||||
| Group C | 4094±411 | 4052±411 | 208±83‡ | 100±62‡ | 96±64‡ | 362±791‡ | 228±259‡ | 1878±1118‡ | 2460±890‡ | 2816±970‡ |
| Group I | 3718±944 | 3253±632* | 138±57‡ | 104±50‡ | 98±38‡ | 80±49‡ | 538±622‡ | 1560±694‡ | 2378±330‡ | 2578±368‡ |
| Group G | 4250±552 | 4364±655† | 188±99‡ | 176±177‡ | 106±99‡ | 146±72‡ | 666±834‡ | 2060±1134‡ | 2710±713‡ | 2990±669‡ |
| Group GI | 4584±757 | 4202±695† | 92±57*‡§ | 56±25‡§ | 44±16‡§ | 222±488‡ | 340±302‡ | 1676±628‡ | 2538±642‡ | 2780±704‡ |
I Ischemia; R1, R5, R10, R20: 1, 5, 10 and 20 min after reperfusion, respectively. P<0.05 compared with the control (C) group;
P<0.05 compared with the isoflurane (I) group;
P<0.05 compared with each value at 0 min (just before ischemia);
P<0.05 compared with the glyburide (G) group. Group GI received glyburide and isoflurane
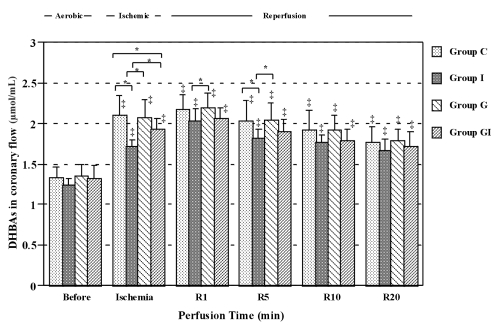
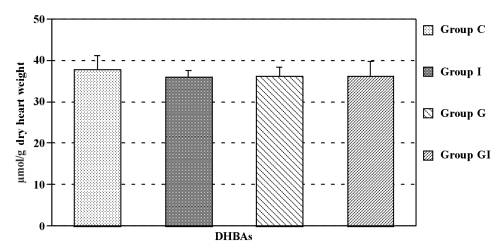
The concentrations of DHBAs in the coronary effluent during ischemia and reperfusion in all groups were significantly higher than those before ischemia. Isoflurane reduced DHBA concentrations significantly in the coronary effluent at ischemia and at some points during the reperfusion period. Glyburide alone had no effect on DHBA concentration. However, in hearts receiving glyburide, isoflurane (group GI) did not decrease DHBA concentrations in the coronary effluent during ischemia and reperfusion (Figure 2). There were no significant differences in myocardial DHBA concentrations between the four groups (Figure 3).
Figure 2).
Dihydroxybenzoic acid (DHBA) concentrations in the perfusate just before ischemia (Before) and just before reperfusion (Ischemia). R1, R5, R10 and R20 are 1, 5, 10 and 20 min after reperfusion, respectively. *P<0.05 compared with each group; ‡P<0.05 compared with before ischemia. Group C Controls; Group G Glyburide; Group GI Glyburide plus isoflurane; Group I Isoflurane
Figure 3).
Concentrations of dihydroxybenzoic acids (DHBAs) in the heart at the end of reperfusion. Group C Controls; Group G Glyburide; Group GI Glyburide plus isoflurane; Group I Isoflurane
DISCUSSION
This study showed that isoflurane reduced hydroxyl radical formation in the postischemic reperfused heart. This result is consistent with our previous finding (11), although there was no difference in myocardial hydroxyl radical content. This is also compatible with another report that halothane prevents postischemic production of hydroxyl radicals in the canine heart (10). These findings suggest that the cardioprotective effects of inhalation anesthetics involve free radicals. On the other hand, activation of KATP channels has been recently reported to be one of the important mechanisms of the cardioprotective effect of isoflurane, which was proved by using the KATP channel blocker glyburide (5).
In the present study, isoflurane’s reducing effect on hydroxyl radicals was blocked by glyburide. Therefore, it is likely that the reducing effect of isoflurane is, at least in part, mediated by isoflurane-induced activation of KATP channels. However, it has been reported that opening of cardiac KATP channels caused hydroxyl radical generation and that glyburide prevented it (15). Moreover, hydroxyl radicals activated KATP channels, although this activation was not prevented by glyburide (16). Conversely, Gan et al (17) have shown that activators of the KATP channel protected against the cardiotoxic properties of hydrogen peroxide, which was inhibited by glyburide. Interestingly, glyburide on its own had no effect on cardiac responses to hydrogen peroxide. Recently, Pain et al (18) found that opening of mitochondrial KATP channels triggered the pre-conditioned state by generating free radicals. Free radicals are known triggers of ischemic preconditioning (19,20). Complicated mechanisms may be involved in the relation between oxygen free radicals and sarcolemmal and mitochondrial KATP channels. In the present study, we used glyburide, which blocks both channels, and did not test the preconditioning effects of isoflurane because we administered them until the end of reperfusion. Further studies are necessary to confirm the relation between isoflurane, KATP channels and free radicals.
We showed that isoflurane decreased the duration of dysrhythmia in the ischemic reperfused heart. Glyburide itself also showed anti-arrhythmic action. In general, KATP channels openers have a tendency to be profibrillatory (21), particularly when the drugs are used at high doses. In contrast, most studies with KATP channel blockers indicate that these drugs tend to be antiarrhythmic (22). However, the mechanism by which KATP blockers produce antiarrhythmic effects has not been clearly elucidated. Barrett et al (23) have suggested that the antiarrhythmic effects of glyburide are not related to KATP blockade in the heart. In the present study, simultaneous administration of glyburide and isoflurane did not have an effect on the duration of dysrhythmia in addition to that of isoflurane or glyburide alone. These findings suggest that the antiarrhythmic action of isoflurane is unlikely to be related to KATP channel activation and may involve other pharmacological actions. Therefore, studies of the cellular mechanisms of isoflurane are necessary to explain the phenomena.
CONCLUSIONS
Our results indicate that isoflurane decreased the duration of dysrhythmia and reduced hydroxyl radical formation in the reperfused heart. Simultaneous administration of glyburide and isoflurane produced no further decrease in the duration of dysrhythmia compared with isoflurane or glyburide alone. The reducing effect of hydroxyl radicals was blocked by glyburide, suggesting that isoflurane reduces hydroxyl radical formation, at least in part, by activating KATP channels.
Acknowledgments
We express our thanks to Mr Koshimizu and Ms Amemia for their valuable technical assistance.
REFERENCES
- 1.Davis RF, Sidi A. Effect of isoflurane on the extent of myocardial necrosis and on systemic hemodynamics, regional myocardial blood flow, and regional myocardial metabolism in dogs after coronary artery occlusion. Anesth Analg. 1989;69:575–86. [PubMed] [Google Scholar]
- 2.Kashimoto S. Effects of isoflurane on myocardial metabolism during postischaemic reperfusion in the rat. Acta Anaesthesiol Scand. 1988;32:199–202. doi: 10.1111/j.1399-6576.1988.tb02714.x. [DOI] [PubMed] [Google Scholar]
- 3.Warltier DC, Al-Wathiqui MH, Kampine JP, Schmeling WT. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology. 1988;69:552–65. doi: 10.1097/00000542-198810000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Oguchi T, Kashimoto S, Yamaguchi T, Nakamura T, Kumazawa T. Comparative effects of halothane, enflurane, isoflurane, and sevoflurane on function and metabolism in the ischaemic rat heart. Br J Anaesth. 1995;74:569–75. doi: 10.1093/bja/74.5.569. [DOI] [PubMed] [Google Scholar]
- 5.Kersten JR, Gross GJ, Pagel PS, Warltier DC. Activation of adenosine triphosphate-regulated potassium channels. Mediation of cellular and organ protection. Anesthesiology. 1998;88:495–513. doi: 10.1097/00000542-199802000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Auchampach JA, Maruyama M, Cavero I, Gross GJ. Pharmacological evidence for a role of ATP-dependent potassium channels in myocardial stunning. Circulation. 1992;86:311–9. doi: 10.1161/01.cir.86.1.311. [DOI] [PubMed] [Google Scholar]
- 7.Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1991;69:571–81. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- 8.Grover GJ, McCullough JR, Henry DE, Conder ML, Sleph PG. Anti-ischemic effects of the potassium channel activators pinacidil and cromakalim and the reversal of these effects with potassium channel blocker glyburide. J Pharmacol Exp Ther. 1989;251:98–104. [PubMed] [Google Scholar]
- 9.Tanguay M, Blaise G, Dumont L, Beique G, Hollman C. Beneficial effects of volatile anesthetics in coronary flow and myocardial contractility induced by oxygen-derived free radicals in isolated rabbit hearts. J Cardiovasc Pharmacol. 1991;18:863–70. doi: 10.1097/00005344-199112000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Glantz L, Ginosar Y, Chevion M, et al. Halothane prevents postischemic production of hydroxyl radicals in the canine heart. Anesthesiology. 1997;86:440–7. doi: 10.1097/00000542-199702000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Kashimoto S, Oguchi T, Kumazawa T. Hydroxyl radical formation during inhalation anesthetics in the reperfused working rat heart. Can J Anaesth. 1999;46:470–5. doi: 10.1007/BF03012948. [DOI] [PubMed] [Google Scholar]
- 12.Neely JR, Liebermeister H, Battersby EJ, Morgan HE. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol. 1967;212:804–14. doi: 10.1152/ajplegacy.1967.212.4.804. [DOI] [PubMed] [Google Scholar]
- 13.Floyd RA, Watson JJ, Wong PK. Sensitive assay of hydroxyl free radical formation utilizing high pressure liquid chromatography with electrochemical detection of phenol and salicylate hydroxylation products. J Biochem Biophys Methods. 1984;10:221–35. doi: 10.1016/0165-022x(84)90042-3. [DOI] [PubMed] [Google Scholar]
- 14.Floyd RA, Henderson R, Watson JJ, Wong PF. Use of salicylate with high pressure liquid chromatography and electrochemical detection (LCED) as a sensitive measure of hydroxyl free radicals in adriamycin treated rats. J Free Radic Biol Med. 1986;2:13–8. doi: 10.1016/0748-5514(86)90118-2. [DOI] [PubMed] [Google Scholar]
- 15.Obata T, Yamanaka Y. Block of cardiac ATP-sensitive K(+) channels reduces hydroxyl radicals in the rat myocardium. Arch Biochem Biophys. 2000;378:195–200. doi: 10.1006/abbi.2000.1830. [DOI] [PubMed] [Google Scholar]
- 16.Tokube K, Kiyosue T, Arita M. Effects of hydroxyl radicals on KATP channels in guinea-pig ventricular myocytes. Eur J Physiol. 1998;437:155–7. doi: 10.1007/s004240050760. [DOI] [PubMed] [Google Scholar]
- 17.Gan XT, Cook MA, Moffat MP, Karmazyn M. Protective effects against hydrogen peroxide-induced toxicity by activators of the ATP-sensitive potassium channel in isolated rat hearts. J Mol Cell Cardiol. 1998;30:33–41. doi: 10.1006/jmcc.1997.0569. [DOI] [PubMed] [Google Scholar]
- 18.Pain T, Yang XM, Critz SD, et al. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–6. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 19.Tritto I, D’Andrea D, Eramo N, et al. Oxygen radicals can induce preconditioning in rabbit hearts. Circ Res. 1997;80:743–8. doi: 10.1161/01.res.80.5.743. [DOI] [PubMed] [Google Scholar]
- 20.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29:207–16. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 21.Wolleben CD, Sanquinetti MC, Siegl PKS. Influence of ATP-sensitive potassium channel modulators on ischemia-induced fibrillation in isolated rat hearts. J Mol Cell Cardiol. 1989;21:783–8. doi: 10.1016/0022-2828(89)90717-7. [DOI] [PubMed] [Google Scholar]
- 22.Hearse DJ. Activation of ATP-sensitive potassium channels: a novel pharmacological approach to myocardial protection? Cardiovasc Res. 1995;30:1–17. [PubMed] [Google Scholar]
- 23.Barrett TD, Walker MJA. Glibenclamide does not prevent action potential shortening induced by ischemia in anesthetized rabbits but reduces ischemia-induced arrhythmias. J Mol Cell Cardiol. 1998;30:999–1008. doi: 10.1006/jmcc.1998.0664. [DOI] [PubMed] [Google Scholar]