Abstract
BACKGROUND:
Terminalia chebula is an ayurvedic drug recommended for the treatment of heart diseases. Earlier studies by the authors validated the beneficial cardioprotective effect of T chebula against isoproterenol-induced myocardial infarction.
OBJECTIVES:
To evaluate the therapeutic efficacy of T chebula in protecting against isoproterenol-induced lysosomal membrane damage.
METHODS:
Lysosomal enzyme activities from the serum, heart and lysosomal fractions were determined. The triphenyltetrazolium chloride assay was used to confirm the protective effect of T chebula on the myocardium.
RESULTS:
Isoproterenol administration produced significant cardiac damage (as seen by the triphenyltetrazolium chloride assay) and significantly altered lysosomal enzyme activities. Pretreatment with an ethanol extract of T chebula was found to retain near normal activities of lysosomal enzymes in rats given T chebula or T chebula plus isoproterenol compared with rats given isoproterenol alone.
CONCLUSIONS:
Pretreatment with T chebula extract stabilizes the lysosomal membrane and, thus, may have prevented myocardial damage.
Keywords: Isoproterenol, Lysosomes, Myocardial injury, Rats, T chebula
Isoproterenol is a synthetic agonist and catecholamine long known to cause severe stress in the myocardium that results in infarct-like myocardial necrosis in rats (1). Isoproterenolinduced necrosis is a multifactorial condition involving relative hypoxia, an effect on coronary microcirculation, membrane permeability and the excessive formation of free radicals. Oxygen free radicals generated during ischemia damage the myocardium through the release of lysosomal enzymes (2). Therefore, isoproterenol-induced myocardial infarction serves as a well-standardized model to study the anti-ischemic effects of Terminalia chebula (Retz), a plant native to India.
According to an ancient treatise on ayurvedha (3), T chebula figures prominently among the list of indigineous remedies advocated for the treatment of cardiac diseases. Studies have demonstrated that T chebula exhibits a wide range of biological activities, including cardioprotective (4), ‘antivata’ or antispasmodic (5), antioxidant (6), free radical scavenging (7) and hypolipidemic properties (8).
Compounds that scavenge for free radicals and have membrane stabilizing potential are reported to be effective in ameliorating the progress of biochemical tissue injury (9). Therefore, the present study sought to evaluate whether pre-treatment with T chebula extract exerts a protective effect against isoproterenol-induced alterations in lysosomal membrane stability and myocardial tissue damage.
METHODS
Chemicals and reagents
Isoproterenol, ethanol, bovine serum albumin, p-nitrophenyl-N-acetyl-beta-D-glucosaminide, p-nitrophenyl-beta-D-glucuronide, p-nitrophenyl-beta-D-glucosaminidase, triphenyltetrazolium chloride (TTC) and p-nitrophenol were obtained from Sigma-Aldrich Company (USA). All other chemicals used were of the highest purity.
T chebula
Powder from the fruit of T chebula was a gift from Rohini Herbal Research Institute Private Limited (Chennai, India). T chebula powder (1 kg) was soaked in 95% ethanol for seven days with intermittent shaking. The solvent was then filtered with Whatman 1 filter paper (Whatman, India). The filtrate was evaporated under a vacuum drier, and the resultant brown residue was stored at −4°C until further use. Weighed amounts of residue were dissolved in 0.9% saline for experimental use.
Animals
Adult male albino rats (Wistar strain) weighing 120 g to 150 g were obtained from Tamilnadu Veterinary and Animal Sciences University, Chennai. The rats were fed with commercial pellet rat chow (Hindustan Lever Limited, India) and given water ad libitum. They were maintained under standard laboratory conditions, with a 12 h light and dark cycle. The study was conducted according to the guidelines of the human/animal ethics committee (University of Madras, India).
Experimental protocol
Preliminary studies were performed to find the dose of T chebula that would be most effective against isoproterenol-induced cardiac damage based on the activities of lactate dehydrogenase (LDH) and creatine kinase. Different doses of T chebula extract, ranging from 250 mg/kg body weight to 1 g/kg body weight were administered at time intervals of 15, 21 and 30 days. The optimal cardioprotective effect of T chebula was observed at a dose of 500 mg/kg body weight for 30 days (data not shown). This dose was therefore used for further studies. The rats were divided into four groups of six rats: ‘normal’ rats (group I); rats administered isoproterenol (200 mg/kg body weight, subcutaneous, given twice with 24 h in-between) (group II); rats pretreated with T chebula extract (500 mg/kg body weight, orally, given daily for 30 days) (group III); and rats pretreated with T chebula extract (500 mg/kg body weight, orally, given daily for 30 days) and administered isoproterenol (200 mg/kg body weight, subcutaneous, given twice with 24 h in-between) at the end of the pretreatment period (group IV).
At the end of the experimental period, the rats were anesthetized with pentobarbital sodium (35 mg/kg body weight, intraperitoneally). Blood was drawn from the external jugular vein and the serum was separated using a Biofuge Stratos centrifuge at 2500 g (Heraeus/Kendro, Germany). The rats were sacrificed 60±5 s after the injection. The hearts were excised, washed in an ice cold 0.9% saline solution, blotted with filter paper and weighed. A section of the heart tissue was used to determine the activities of lysosomal enzymes.
Lysosomal fractions were isolated using the method of Wattiaux (10). The activities of the lysosomal enzymes were determined for beta-D-glucuronidase using the method of Hultberg et al (11); beta-D-glucosidase using the method of Conchie et al (12); beta-N-acetyl-glucosaminidase using the method of Moore and Moris (13); cathepsin D using the method of Sapolsky et al (14); and acid phosphatase using the method of King (15). The lysosome pellet was suspended in 1.15% KCl and used for the estimation of enzyme activity.
TTC assay
A section of the heart tissue was used for the TTC assay as described by Lie et al (16). The myocardium of the rat was frozen immediately after removal. The ventricle portion of the heart was excised, weighed, sliced into 1 mm segments and incubated in a 1% TTC solution at 37°C for 20 min. The weight of the infarcted tissue was expressed as a percentage of the total ventricular weight.
Statistical analysis
The data were analysed using one-way ANOVA followed by Bonferroni’s multiple comparison test. The results from the experimental groups were compared with their respective control group. P<0.05 was considered statistically significant. The infarct size was analysed using one-way ANOVA followed by Student’s t test, and P<0.001 was considered statistically significant.
RESULTS
The activities of lysosomal hydrolases in the sera of control and experimental groups are shown in Table 1. Significant elevations in the activities of beta-D-glucuronidase, beta-D-glucosidase, beta-N-acetyl-glucosaminidase, cathepsin D and acid phosphatase were observed in isoproterenol-administered rats (group II) compared with the control rats (group I). In isoproterenol-administered rats pretreated with T chebula extract (group IV), significantly lower activities in serum lysosomal hydrolases were observed compared with rats injected with isoproterenol alone (group II) (P<0.01).
TABLE 1.
Effect of Terminalia chebula extract on the activities of lysosomal enzymes in the sera of control and experimental rats
| Group I (control) | Group II (isoproterenol injected) | Group III (T chebula) | Group IV (T chebula plus isoproterenol) | ANOVA F value | |
|---|---|---|---|---|---|
| Beta-D-glucuronidase* | 8.90±0.70 | 14.00±1.07§ | 8.50±0.44¶ | 10.03±0.91** | 50.21 |
| Beta-D-glucosidase* | 9.01±0.33 | 15.01±0.97§ | 9.01±0.81¶ | 10.80±1.50** | 48.20 |
| Beta-N-acetyl-glucosaminidase* | 18.00±1.30 | 27.30±2.12§ | 17.50±0.75¶ | 25.80±2.19†† | 53.70 |
| Cathepsin D† | 14.00±1.21 | 20.00±2.40§ | 13.91±1.24¶ | 16.08±1.29** | 18.34 |
| Acid phosphatase‡ | 80.23±8.80 | 112.25±8.86§ | 78.26±7.65¶ | 89.11±7.90** | 21.02 |
Enzyme activity expressed in:
p-nitrophenol liberated (μmol/h/100 mg protein);
Tyrosine liberated (μmol/h/100 mg protein); and
p-nitrophenol liberated (μmol/min/100 mg protein).
Significantly different compared with group I (P<0.01);
Not statistically different compared with group I.
Significantly different compared with group II (P<0.01).
Significantly different compared with group II (P<0.05) (Bonferroni’s Multiple Comparison Test). Values are expressed as mean ± SD for six rats in each group
The activities of the lysosomal hydrolases from heart tissue homogenates of the control and experimental groups are shown in Table 2. Significant increases in the activities of beta-D-glucuronidase, beta-D-glucosidase, beta-N-acetyl-glucosaminidase, cathepsin D and acid phosphatase were observed in the heart tissue of isoproterenol-administered rats compared with the control rats (group I) (P<0.01). T chebula extract pretreatment (group IV), however, resulted in significantly lower activities of heart lysosomal enzymes compared with rats given isoproterenol alone (group II).
TABLE 2.
Effect of Terminalia chebula extract on the activities of lysosomal enzymes in the heart homogenate of control and experimental rats
| Group I (control) | Group II (isoproterenol injected) | Group III (T chebula) | Group IV (T chebula plus isoproterenol) | ANOVA F value | |
|---|---|---|---|---|---|
| Beta-D-glucuronidase* | 22.43±2.94 | 34.88±5.29§ | 23.23±3.29¶ | 25.36±3.18** | 13.69 |
| Beta-D-glucosidase* | 15.20±1.01 | 28.20±1.41§ | 15.12±1.03¶ | 19.39±0.58** | 204.74 |
| Beta-N-acetyl-glucosaminidase* | 47.64±4.42 | 67.63±7.89§ | 43.30±4.17¶ | 50.27±7.52** | 17.02 |
| Cathepsin D† | 28.75±3.88 | 46.34±3.75§ | 27.22±3.39¶ | 33.56±2.92** | 36.68 |
| Acid phosphatase‡ | 120.03±11.82 | 155.48±14.36§ | 113.52±12.00¶ | 135.48±14.36** | 4.53 |
Enzyme activity expressed in:
p-nitrophenol liberated (μmol/h/100 mg protein);
Tyrosine liberated (μmol/h/100 mg protein); and
p-nitrophenol liberated (μmol/min/100 mg protein).
Significantly different compared with group I (P<0.01);
Not statistically different compared with group I.
Significantly different compared with group II (P<0.01) (Bonferroni’s Multiple Comparison Test). Values are expressed as mean ± SD for six rats in each group
The activities of lysosomal hydrolases in the lysosomal fractions of the control and experimental groups are shown in Table 3. The activities of lysosomal hydrolases were found to be significantly decreased in the lysosomal fraction of group II rats compared with group I rats. The activities of these enzymes were maintained at near normal levels in rats pretreated with T chebula extract (group IV). Rats pretreated with T chebula extract alone (group III) showed no significant change in lysosomal hydrolases activities in the serum, heart and lysosomal fractions compared with controls.
TABLE 3.
Effect of Terminalia chebula extract on the activities of lysosomal enzymes in the lysosomal fraction of control and experimental rats
| Group I (control) | Group II (isoproterenol injected) | Group III (T chebula) | Group IV (T chebula plus isoproterenol) | ANOVA F value | |
|---|---|---|---|---|---|
| Beta-D-glucuronidase* | 41.94±5.10 | 22.88±2.64§ | 42.58±4.66** | 37.58±3.48†† | 30.23 |
| Beta-N-acetyl-glucosaminidase* | 52.38±5.47 | 31.66±4.00§ | 49.63±4.47** | 45.55±4.45†† | 23.66 |
| Cathepsin D† | 70.55±7.56 | 58.38 ±5.44¶ | 69.76±6.77** | 67.26±7.43‡‡ | 5.03 |
| Acid phosphatase‡ | 121.36±10.82 | 83.39±5.38§ | 122.53±11.04** | 105.03±11.75†† | 19.51 |
Enzyme activity expressed in:
p-nitrophenol liberated (μmol/h/100 mg protein);
Tyrosine liberated (μmol/h/100 mg protein); and
p-nitrophenol liberated (μmol/min/100 mg protein).
Significantly different compared with group I (P<0.01).
Significantly different compared with group I (P<0.05).
Not statistically different compared with group I.
Significantly different compared with group II (P<0.01).
Significantly different compared with group II (P<0.05) (Bonferroni’s Multiple Comparison Test). Values are expressed as mean ± SD for six rats in each group
TTC assay
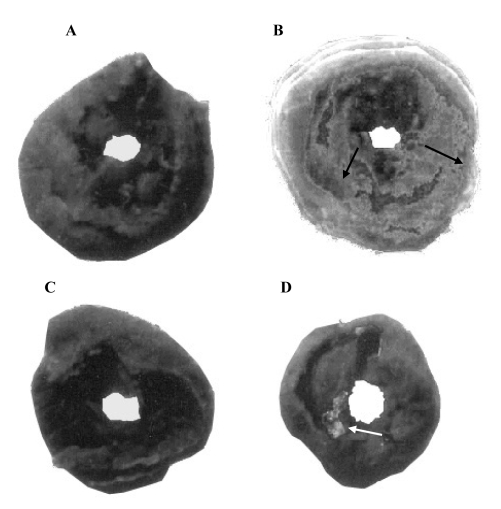
TTC macroscopic enzyme-mapping assay of sections of heart from control and experimental rats (Figure 1) is direct evidence of myocardial necrosis. Figure 1A shows a section of heart from a control rat with viable myocardial tissue stained to indicate the presence of LDH and intact myocardial tissue. Figure 1B shows a section of heart from an isoproterenol-administered rat. Necrotic tissues are clearly visible as light gray patches. One of the characteristic features of isoproterenol administration is the loss of LDH activity from myocardium, and may reflect the consequence of cellular injury. Figure 1C, a section of heart tissue from a rat pre-treated with T chebula extract alone, shows results similar to that of the control. Figure 1D shows a section of heart tissue from a T chebula-pretreated rat administered isoproterenol. A major portion of the heart tissue stained positive for viability, indicating that the prior oral administration of T chebula extract may have prevented membrane damage by isoproterenol, thereby retaining near normal myocardial membrane structural and functional integrity. The effect of T cheblua on myocardial infarct size of control and experimental rats is shown in Table 4.
Figure 1).
Representative results from the triphenyltetrazolium chloride assay of sections of heart from the control (n=6) and experimental rat (n=6) groups: (A) Control rats (group I); (B) Isoproterenol-administered rats (group II); (C) Terminalia chebula-pretreated rats (group III); (D) T chebula-pretreated plus isoproterenol-administered rats (group IV). Arrows indicate patches of necrotic tissue
TABLE 4.
Effect of Terminalia chebula extract on the myocardial infarct size of control and experimental rats
| Necrotic tissue (% of ventricle) | ANOVA F value | |
|---|---|---|
| Group I (control) | 0.00±0 | |
| Group II (isoproterenol injected) | 28.1±2.8* | 131.3 |
| Group III (T chebula) | 0.00±0 | |
| Group IV (T chebula plus isoproterenol) | 9.1±0.3 |
Statistically significant from group I (P<0.001; one-way ANOVA followed by Student’s t test). Values are expressed as mean ± SD for six rats in each group
DISCUSSION
Lysosomal enzymes are important mediators of acute and chronic inflammatory diseases, and can cause damage to connective tissue (17). Alterations in the activity of lysosomal enzymes have been observed in patients with myocardial infarction (18) and in experimental animal models (19). Therefore, considerable attention has been focused on lysosomal enzyme alterations that may accompany ischemic or hypoxic myocellular damage (20,21).
Macickova et al (22) observed that isoproterenol is able to induce changes in lysosomal enzyme activity both in vivo and in vitro. Isoproterenol-induced myocardial infarction results in increased activities of the lysosomal hydrolases in the serum and heart tissue, and a decrease in their activities in the lysosomal fraction. This may be responsible for myocardial cellular injury and death in the ischemic state of the heart (22,23). The findings in the present study support this.
It has been previously reported that ischemia produces rapid accumulation of lactic acid and other metabolic acids (which lowers intracellular pH), and decreases in ATP and the active accumulation of free fatty acids (25,26). In turn, these events produce a reduction in membrane integrity, which initiates the release of lysosomal enzymes. The leakage of the enzymes from the enclosed sacs leads to intracellular dysfunction, disruption of potential substrates (27) and organelles (mitochondria [28], sarcolemma, etc [29,30]), and autolysis of myocardial cells (31). The significantly lower activities of the enzymes in the lysosomal fraction in isoproterenol-administered rats correlates well with these findings. T chebula extract pretreatment led to the retainment of near normal activity of the enzymes in the lysosomal fraction, suggesting the stabilization of the lysosomal membrane by T chebula extract.
The increased activities of cathepsin D and glycohydrolases in heart tissue indicate the possible infiltration of inflammatory cells at the site of infarction. During myocardial infarction, when myocardial cell death and degeneration occurs, proteolysis of necrotic myocardium occurs with a concomitant influx of inflammatory cells at the infarct margins (32).
In experimental myocardial infarction, decreased lysosomal stability leads to elevated levels of lysosomal enzymes in the extracellular fluid (33), and alters the metabolism of different connective tissue constituents, namely, glycosaminoglycans and glycoproteins (34). The release of lysosomal enzymes contributes to tissue destruction and may be one of the causes of increased focal lesions observed in heart tissue (35). In the present study, the observed increases in the activities of lysosomal enzymes in the serum and heart tissue is an indication of isoproterenol-mediated lysosomal membrane damage.
The release of lysosomal enzymes into the cytoplasm stimulates inflammatory mediators (eg, oxygen radicals and prostaglandin), which then stimulate tissue disruption. Considering this and the well-known lytic action of lysosomal enzymes, it suggests that the damage to the lysosomal membrane and alterations in the fragility of lysosomes may be among the earliest structural alterations that occur during the development of ischemic myocardial injury (22).
Pretreatment with orally administered T chebula extract led to the retention of near normal activities of the lysosomal enzymes in the serum and heart tissue. Pretreatment with T chebula extract was associated with a decreased release of enzymes from the lysosomal fractions, which could be due to the membrane stabilizing effect of T chebula on the lysosomal membrane. T chebula has been reported to possess higher anti-inflammatory activity compared with prednisolone (36). T chebula has been reported to possess flavonoids which exhibit anti-inflammatory, vasodilatory, lipid peroxidation, antioxidant and free radical scavenging properties (37–39). The anti-oxidant property is due to T chebula extract scavenging for oxygen free radicals, resulting in the preservation of cellular viability serving, secondarily, to preserve lysosomes and, thereby, retaining near normal functioning of the lysosomes.
CONCLUSIONS
Pretreatment with T chebula extract may partly impart its cardioprotective effect through lysosomal membrane stabilization, thus preventing myocardial necrosis.
Acknowledgments
S Suchalatha wishes to thank the Council of Scientific and Industrial Research for the financial assistance granted in the form of a Senior Research Fellowship.
REFERENCES
- 1.Rona G, Chappel CI, Balazs T, Gaudry R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch Pathol. 1959;67:443–55. [PubMed] [Google Scholar]
- 2.Kalra J, Prasad K. Oxygen free radicals and cardiac depression. Clin Biochem. 1994;27:163–8. doi: 10.1016/0009-9120(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 3.Nadkarni KM. Revised and enlarged by AK MadKarni, 1956. Bombaby: Bombay Popular Prakashan PVP; 1976. Indian Materia Medica, with Ayurvedic, Unani and Home Remedies; p. 1207. [Google Scholar]
- 4.Suchalatha S, Shyamala Devi CS. Protective effect of Terminalia chebula against experimental myocardial injury induced by isoproterenol. Indian J Exp Biol. 2004;42:174–8. [PubMed] [Google Scholar]
- 5.Hashimoto M, Nakajima Y. Antiobesity agents, alpha-amylase inhibitors, lipase inhibitors, foods and beverages containing plant extracts. Jpn Kokai Tokyo Koho JP. 1997;227:398. [Google Scholar]
- 6.Kato Y, Nagao A, Terao J, Osawa T. Inhibition of mycloperoxidase-catalyzed tyrosylation by phenolic antioxidants in vitro. Biosci Biotechnol Biochem. 2003;67:1136–9. doi: 10.1271/bbb.67.1136. [DOI] [PubMed] [Google Scholar]
- 7.Cheng HY, Lin TC, Yu KH, Yang CM, Lin CC. Antioxidant and free radical scavenging activities of Terminalia chebula. Biol Pharm Bull. 2003;26:1331–5. doi: 10.1248/bpb.26.1331. [DOI] [PubMed] [Google Scholar]
- 8.Shaila HP, Udupa SL, Udupa AL. Hypolipidemic activity of three indigenous drugs in experimentally induced atherosclerosis. Int J Cardiol. 1998;67:119–24. doi: 10.1016/s0167-5273(98)00281-2. [DOI] [PubMed] [Google Scholar]
- 9.Munasinghe TC, Seneviratne CK, Thabrew MI, Abeysekera AM. Antiradical and antilipoperoxidative effects of some plant extracts used by Sri Lankan traditional medical practitioners for cardioprotection. Phytother Res. 2001;15:519–23. doi: 10.1002/ptr.994. [DOI] [PubMed] [Google Scholar]
- 10.Wattiaux R. Lysosomal membranes. In: Jamieson GA, Robinson DM, editors. Mammalian Cell Membranes. London: Buttersworths and Co; 1977. p. 165. [Google Scholar]
- 11.Hultberg B, Lindsten J, Sjoblad S. Molecular forms and activities of glycosidases in cultures of amniotic-fluid cells. Biochem J. 1976;155:599–605. doi: 10.1042/bj1550599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conchie J, Gelman AL, Levvy GA. Inhibition of glycosidases by aldonolactones of corresponding configuration. The C-4- and C-6- specificity of beta-glucosidase and beta-galactosidase. Biochem J. 1967;103:609–15. doi: 10.1042/bj1030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore JC, Morris JE. A simple automated colorimetric method for determination of N-acetyl-beta-D-glucosaminidase. Ann Clin Biochem. 1982;19:157–9. doi: 10.1177/000456328201900305. [DOI] [PubMed] [Google Scholar]
- 14.Sapolsky AI, Altman RD, Howell DS. Cathepsin D activity in normal and osteoarthritic human cartilage. Fed Proc. 1973;32:1489–93. [PubMed] [Google Scholar]
- 15.King J. The hydrolases – acid and alkaline phosphatases. In: Van D, editor. Practical Clinical Enzymology. London: Nostrand; 1965. pp. 199–208. [Google Scholar]
- 16.Lie JT, Pairolero PC, Holley KE, Titus JL. Macroscopic enzyme mapping verification of large, homogenous, experimental myocardial infarcts of predictable size and location in dogs. J Thorac Cardiovasc Surg. 1975;69:599–605. [PubMed] [Google Scholar]
- 17.Wildenthal K, Decker RS, Poole AR, Griffin EE, Dingle JT. Sequential lysosomal alterations during cardiac ischemia. I. Biochemical and immunohistochemical changes. Lab Invest. 1978;38:656–61. [PubMed] [Google Scholar]
- 18.Welman E, Selwyn AP, Peters TJ, Colbeck JF, Fox KM. Plasma lysosomal enzyme activity in acute myocardial infarction. Cardiovasc Res. 1978;12:99–105. doi: 10.1093/cvr/12.2.99. [DOI] [PubMed] [Google Scholar]
- 19.Mathew S, Menon PV, Kurup PA. Metabolism of glycosaminoglycans in the heart of rats subjected to isoproterenol-induced myocardial infarction. Indian J Biochem Biophys. 1982;19:352–5. [PubMed] [Google Scholar]
- 20.Frolov VA, Kapustin VA. [Effect of vitamin A and E on the contractile function of the heart in experimental myocardial infarction] Kardiologiia. 1983;23:93–5. [PubMed] [Google Scholar]
- 21.Remla A, Menon PV, Kurup PA. Effect of ethanol administration on changes in metabolism of glycosaminoglycans in heart & aorta in isoproterenol-induced myocardial infarction in rats. Indian J Biochem Biophys. 1983;20:295–300. [PubMed] [Google Scholar]
- 22.Macickova T, Navarova J, Urbancikova M, Horakova K. Comparison of isoproterenol-induced changes in lysosomal enzyme activity in vivo and in vitro. Gen Physiol Biophys. 1999;18:86–91. [PubMed] [Google Scholar]
- 23.Ravichandran LV, Puvanakrishnan R, Joseph KT. Influence of isoproterenol-induced myocardial infarction on certain glycohydrolases and cathepsins in rats. Biochem Med Metab Biol. 1991;45:6–15. doi: 10.1016/0885-4505(91)90003-4. [DOI] [PubMed] [Google Scholar]
- 24.Nirmala C, Puvanakrishnan R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol Cell Biochem. 1996;159:85–93. doi: 10.1007/BF00420910. [DOI] [PubMed] [Google Scholar]
- 25.Neely JR, Denton RM, England PJ, Randle PJ. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem J. 1972;128:147–59. doi: 10.1042/bj1280147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta MP, Manchanda SC, Seth SD. Biochemical basis of the protective effect of propanalol pretreatment in experimental myocardial necrosis. Indian J Exp Biol. 1982;20:79–83. [PubMed] [Google Scholar]
- 27.Decker RS, Wildenthal K. Sequential lysosomal alterations during cardiac ischemia. II. Ultrastructural and cytochemical changes. Lab Invest. 1978;38:662–73. [PubMed] [Google Scholar]
- 28.Kennett FF, Weglicki WB. Effects of well-defined ischemia on myocardial lysosomal and microsomal enzymes in a canine model. Circ Res. 1978;43:750–8. doi: 10.1161/01.res.43.5.750. [DOI] [PubMed] [Google Scholar]
- 29.Gottwik MG, Kirk ES, Kennett FF, Weglicki WB. Release of lysosomal enzymes during ischemic injury of canine myocardium. Recent Adv Stud Cardiac Struct Metab. 1976;12:431–8. [PubMed] [Google Scholar]
- 30.Ogawa T, Hieda N, Sugiyama S, et al. Effect of a novel thromboxane A2 synthetase inhibitor on ischemia-induced mitochondrial dysfunction in canine hearts. Arzneimittelforschung. 1988;38:228–30. [PubMed] [Google Scholar]
- 31.Niebes P, Ponard G. Stabilization of rat liver lysosomes by (+)-cyanidanol-3 in vivo. Biochem Pharmacol. 1975;24:905–9. doi: 10.1016/0006-2952(75)90162-8. [DOI] [PubMed] [Google Scholar]
- 32.Mathew S, Menon PV, Kurup PA. Changes in glycoproteins in isoproterenol-induced myocardial infarction in rats. Indian J Biochem Biophys. 1982;19:41–3. [PubMed] [Google Scholar]
- 33.Kumar JS, Menon VP. Changes in levels of lipid peroxides and activity of superoxide dismutase and catales in diabetes associated with myocardial infarction. Ind J Exp Biol. 1992;30:122–7. [PubMed] [Google Scholar]
- 34.Decker RS, Wildenthal K. Role of lysosomes and latent hydrolytic enzymes in ischemic damage and repair of the heart. In: Wildenthal K, editor. Degradative Processes in Heart and Skeletal Muscle. Amsterdam: Elsevier/North Holland Biomedical Press; 1980. pp. 389–418. [Google Scholar]
- 35.Ebenezar K, Sathish V, Devaki T. Effect of L-arginine and L-lysine on lysosomal hydrolases and membrane bound phosphatases in experimentally induced myocardial infarction in rats. Mol Cell Biochem. 2003;247:163–9. doi: 10.1023/a:1024115714236. [DOI] [PubMed] [Google Scholar]
- 36.Sharma AK, Singh RH. Screening of the anti-inflammatory activity of certain indigenous drugs on carageenin induced hind paw oedema. Bull Med Ethnobot Res. 1980;1:262–71. [Google Scholar]
- 37.Hanaski Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16:845–50. doi: 10.1016/0891-5849(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 38.Duarte J, Perez Vizcaino F, Utrilla P, Jimenez J, Tamargo J, Zarzuelo A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen Pharmacol. 1993;24:857–62. doi: 10.1016/0306-3623(93)90159-u. [DOI] [PubMed] [Google Scholar]
- 39.Miyagi Y, Miwa K, Inoue H. Inhibition of human low-density lipoprotein oxidation by flavonoids in red wine and grape juice. Am J Cardiol. 1997;80:1627–31. doi: 10.1016/s0002-9149(97)00755-8. [DOI] [PubMed] [Google Scholar]