Figure 1.
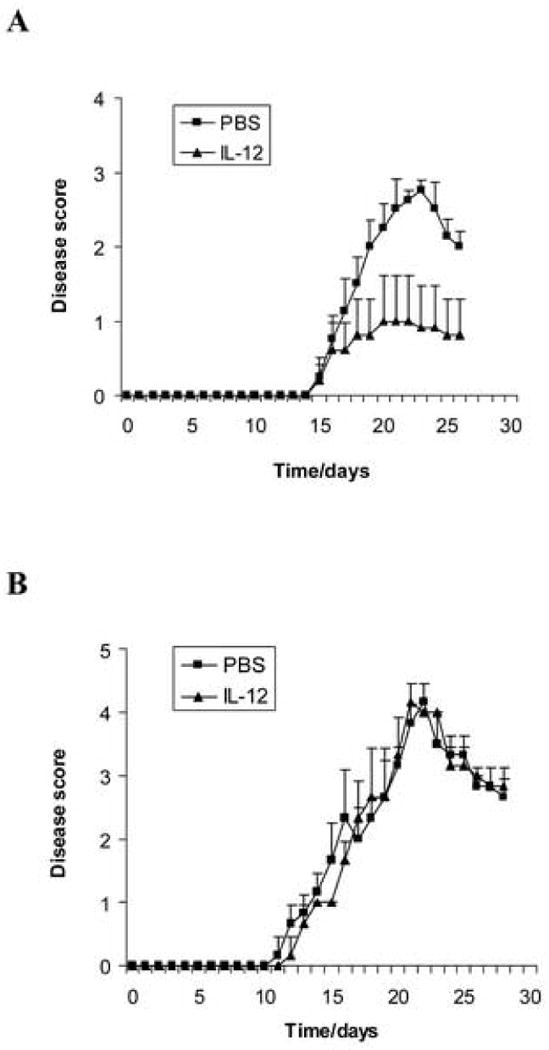
IL-12 suppresses EAE in wild type mice but not in IFN-γ-deficient mice. Female C57BL/6 wild type mice (A) and IFN-γ-deficient mice (B) were immunized with 300 μg MOG35-55 peptide in CFA. The number of mice in each group was 5. rmIL-12 (100 ng/mouse, dissolved in 200 μl PBS) was injected intraperitoneally (i.p.) every day from day 0 to day 10 post-immunization. PBS (200 μl/mouse) only was injected i.p. as control. Clinical disease severity of EAE was scored daily by double blinded method according to a 0-5 severity scale. Data represent the mean clinical scores ± SEM. There is a significant difference in clinical disease score of EAE between the rmIL-12-treated group and the PBS control group in C57BL/6 wild type mice (p<0.01) but there is no significant difference between the two groups in IFN-γ-deficient mice (p>0.05). Experiments were repeated three times and similar results were obtained. One representative experiment is shown.
