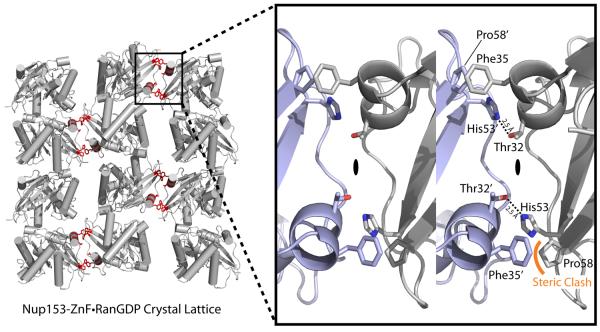
Figure 3.
Crystallographic lattice formed in the crystallization of a Nup153-ZnF•RanGDP complex. Residues highlighted in red indicate contacts made between neighboring Ran molecules down a single plane of the P21 lattice. This plane represents the smallest crystallographic interface and was chosen for crystal engineering to stabilize packing, in hopes of improving diffraction. The enlarged 3D image on the right depicts residues binding at the interface. Thr32' and His53 make a single hydrogen bond, mirrored across a 2-fold symmetrical face as indicated. A clash between Phe35' and Pro58, though tolerated, was theorized to partially counteract the strong interaction between Thr32' and His53. A point-mutation of Phe35' to Serine stabilizes the crystal contact and increases the resolution of our crystallographic experiments by 1 Å.