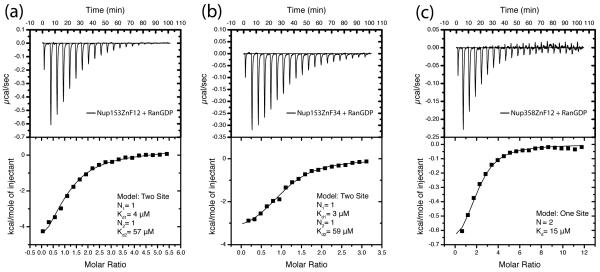
Figure 7.
ITC illustrating low μM affinity between RanGDP and two tandem ZnF pairs from the Nup153-and Nup358-ZnF domain. (a) Interaction between RanGDP and ZnF12. The data is best fit to a two-site model suggesting each ZnF binds independently, but with different affinities. (b) Interaction between ZnF34 and RanGDP, again suggesting two independent sites with different affinities. (c) Interaction between Nup358ZnF12 and RanGDP. As with Nup153, the ZnFs of Nup358 bind with low μM affinity to RanGDP. The data is best fit to a single site model in accordance with both ZnFs from Nup358 lacking the residue nececcary at pos8 to H-bond with Gln10Ran. Experimental values for N, Kd, enthalpy, ΔH, and entropy, TΔS, are listed in Table 2. Constructs are described in Supplementary Table 1.