Figure 2.
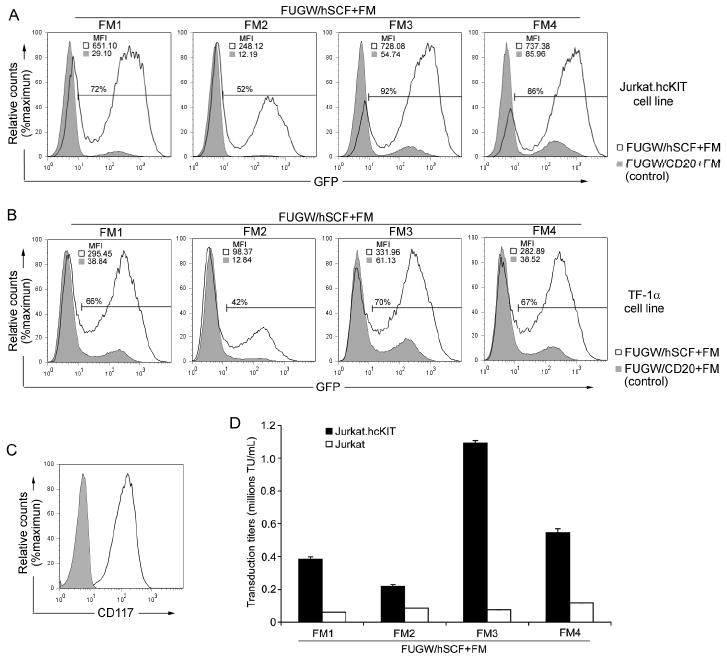
Engineered lentiviral vector transduction of targeted cells in vitro. (A&B) Fresh unconcentrated recombinant lentiviral vectors (2 mL) either bearing: hSCF and the indicated fusogen (FUGW/hSCF+FM) (solid line) or CD20 (control ligand, no specificity to CD117) and the indicated fusogen (FUGW/CD20+FM) (shaded area), were added to CD117-expressing Jurkat.hcKIT cells (A) or TF-1α (B). Three days post-transduction the resulting eGFP expression was taken as an indication of transduction efficiency and mean fluorescence intensity was analyzed by flow cytometry with cell counts normalized and a representative result of fluorescence shown with gating of FUGW/hSCF+FM transduced cells set using non-infected cells. (C) Flow cytometry analysis of the Jurkat.hcKIT cell line by surface staining using anti-CD117 antibody. Solid line: CD117 expression on Jurkat.hcKIT cell line; Shaded area: CD117 expression on Jurkat cell line (as a control). (D) Specific viral titers for various engineered lentiviral vectors. Fresh unconcentrated lentiviral vectors incorporating hSCF and a fusogen were used to transduce (2 × 104) Jurkat.hcKIT or Jurkat cells in a 96 well plate. The viral titer was conducted in duplicate and measured in dilution ranges that exhibited a linear response of eGFP expression with viral dilution.
