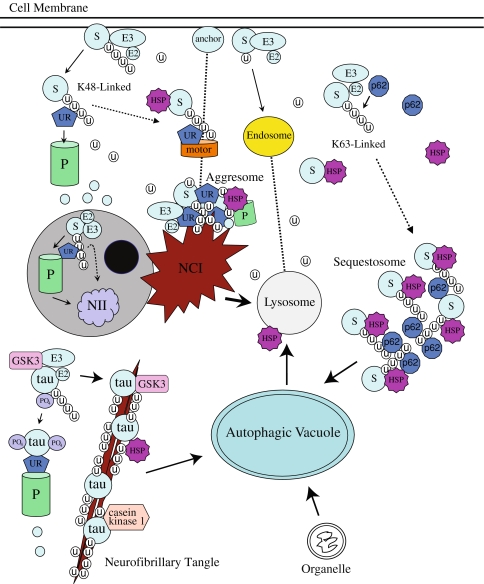
Fig. 4.
Simplified model of the UPS, endosome–lysosome system and autophagy cellular pathways. Cell membrane proteins that are UPS substrates (S) are generally monoubiquitinated and degraded in the endosome–lysosome system, or polyubiquitinated and delivered to the 26S proteasome by ubiquitin receptor proteins (UR). Nuclear proteins are degraded in the proteasome within the nucleus or translocated to the cytosol where they can be degraded by various routes. Polyubiquitinated nuclear proteins may be deposited into neuronal intranuclear inclusions (NII) in disease states. Polyubiquitinated cytosolic proteins and UPS machinery proteins such as E2s, E3s and proteasomal subunits may be transported along microtubules (dotted lines) to be deposited into aggresomes, which may form neuronal cytoplasmic inclusions (NCI). Similar to other SCF E3 ligase substrates, tau is probably first phosphorylated by casein kinase 1, which facilitates further phosphorylation by GSK3. Hyperphosphorylated tau is deposited into neurofibrillary tangles, which like NCIs may be cleared by autophagy. K63-linked polyubiquitinated chains bind p62 and form complexes known as sequestosomes. Aggregation of insoluble misfolded proteins is prevented by heat shock proteins (HSP), which also promote the fusion of sequestosomes to autophagic vacuoles and ultimately the lysosome