Figure 1.
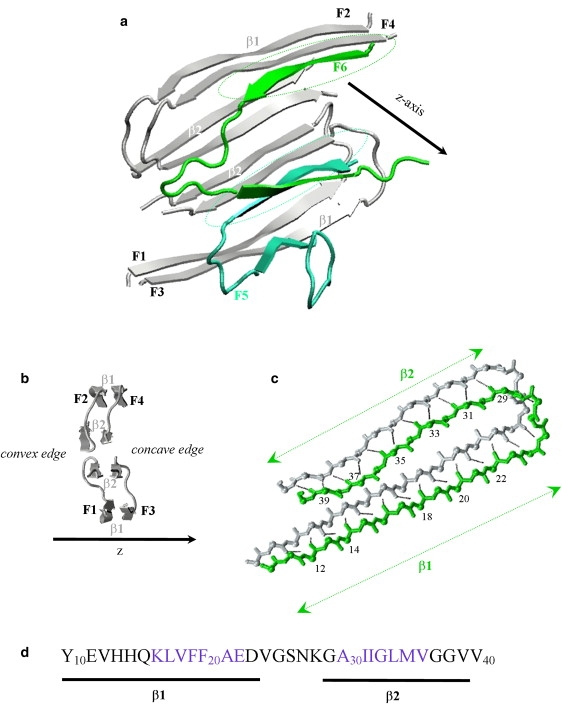
(a) Cartoon backbone representation of Aβ10–40 hexamer used in this study. Aβ peptides F1-F4 (in gray) represent fibril fragment derived from solid-state NMR measurements (11). Fibril protofilament consists of four laminated β-sheets formed by the β1 and β2 strands in Aβ sequence (see panel d). The incoming peptides F5 and F6 (in aqua and green) are docked to the fibril edge. Two β strands in incoming peptides form parallel off-registry β-sheets with the fibril (marked by dashed stretched circles), which constitute the emerging locked phase. The fibril axis is parallel to the z axis. (b) Lateral view on the Aβ fibril fragment shown in panel a. Due to the stagger of β2 sheets with respect to β1, Aβ fibril has two distinct edges—concave and convex. (c) Parallel in-registry alignment of the fibril peptide (in gray) and the edge peptide (in green) on the concave fibril edge. This structure is typical for the peptides in the fibril interior. Backbone HBs are shown by black dashed lines. The indices of the residues in the edge peptide engaged in HBs and allocation of β1 and β2 strands are shown. Panels a–c are prepared using VMD (73). (d) The sequence of Aβ10–40 monomer and the allocation of the β1 and β2 segments, which participate in fibril β-sheets (see panel a). The residues in blue have the highest propensity to form β-sheet structure according to NPS consensus prediction tool (74). Central hydrophobic cluster includes the residues 17–21 in β1. The color version of this figure is available online.
