Figure 4.
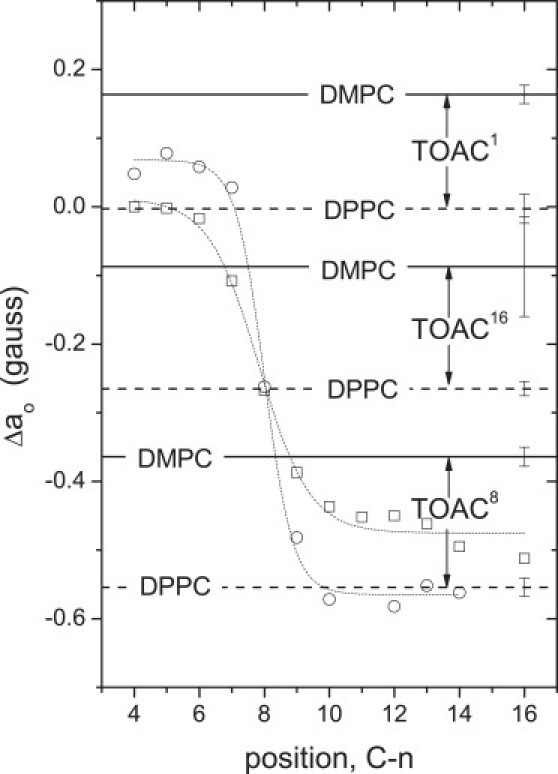
Effective isotropic hyperfine couplings, ao, for [Glu(OMe)7,18,19] alamethicin TOAC1, TOAC8, and TOAC16 analogs, as indicated, in fluid-phase DMPC (solid horizontal lines) and fluid-phase DPPC (dashed horizontal lines) bilayers (8,9). To establish the transmembrane location of the TOAC labels, the dependence of ao on nitroxide position, n, in the sn-2 chain of spin-labeled phosphatidylcholine, n-PCSL, in DMPC (circles) and DPPC (squares) fluid-phase bilayers is given by the dotted lines (10). All values of ao are given relative to those in methanol, i.e.: Δao = ao(PC) − ao(MeOH), and are constant over a wide range of temperature in the respective fluid phases. The relative order for Δao of TOACm-Alm in fluid-phase phosphatidylcholines is: TOAC1 > TOAC16 > TOAC8, for chain lengths C12–C18 (9).
