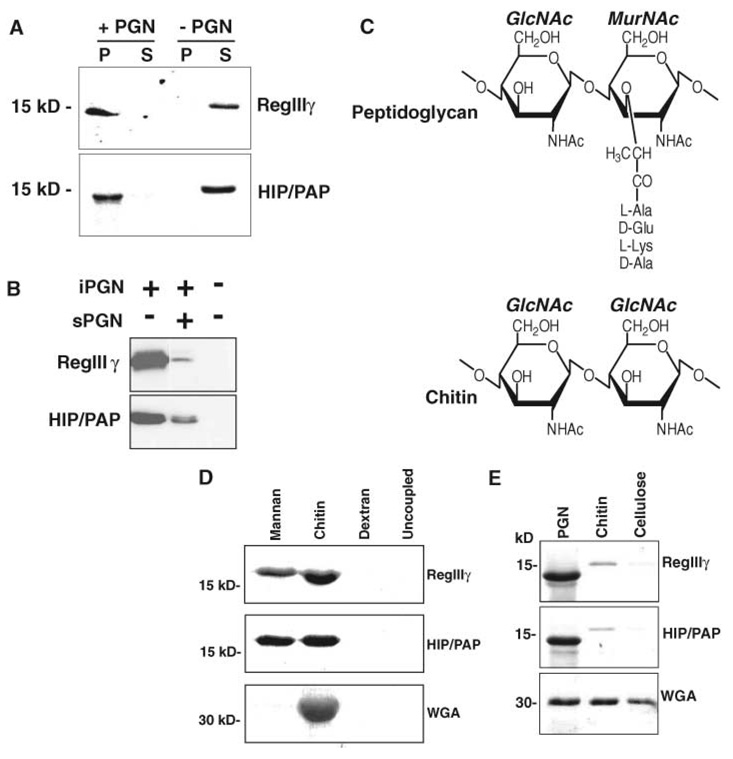
Fig. 2.
Mouse RegIIIγ and human HIP/PAP bind peptidoglycan. (A) Peptidoglycan pull-down assays. RegIIIγ or HIP/PAP (10 µg of either) was added to 50 µg insoluble Bacillus subtilis peptidoglycan and pelleted. Pellet (P) and supernatant (S) fractions were analyzed by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining. (B) sPGN competes with iPGN for lectin binding. Pull-down assays were performed with or without 100 µM soluble B. subtilis peptidoglycan. (C) Comparison of peptidoglycan and chitin structures. The structure of a typical Gram-positive peptidoglycan is depicted. (D) Lectin binding to immobilized polysaccharides. Lectins were bound to immobilized polysaccharide for 2 hours at 4°C. After washing, bound proteins were released by boiling in SDS-PAGE sample buffer and analyzed by SDS-PAGE and Coomassie blue staining. (E) Pull-down assays comparing binding to peptidoglycan, chitin, and cellulose. Purified recombinant RegIIIγ or HIP/PAP (10 µg of either) was added to 50 µg of peptidoglycan, chitin, or cellulose and analyzed as in (A). The lower molecular weight forms of RegIIIγ and HIP/PAP in (A) and (E) result from cleavage at an N-terminal trypsinlike site by a peptidoglycan-associated proteolytic activity.