Figure 4.
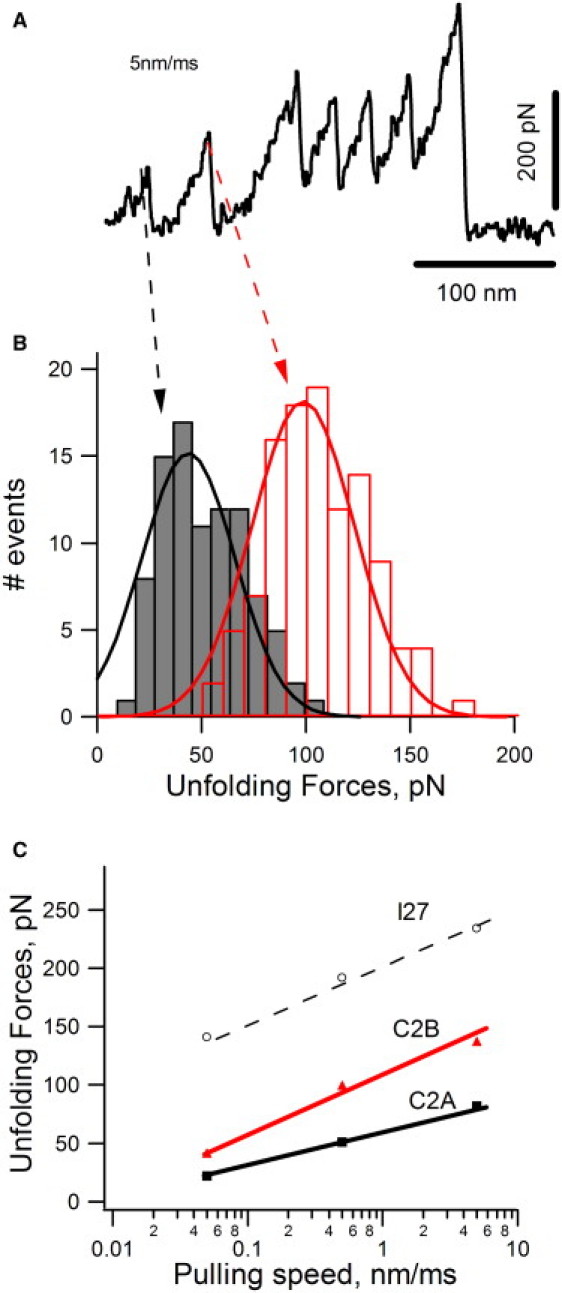
Mechanical properties of C2A versus C2B domains. (A) Force-extension curve obtained after stretching a I272-C2AB-I272 protein at 5 nm/ms. We identified the first peak as the mechanical unfolding of the C2A domain and the second as the unfolding of the C2B domain. (B) Unfolding force histogram for C2A (black) and C2B (red) domains. The average unfolding forces are ∼50 pN (49 ± 18 pN, n = 91) and ∼100 pN (106 ± 23 pN, n = 110), respectively. (C) Plot of unfolding forces versus loading rate for C2A (black squares), C2B (red triangles), and I27 (open circles). A 100-fold increase in loading rate increases the unfolding forces by 60 pN for C2A, 96 pN for C2B, and 93 pN for I27.
