Abstract
During cardiac ischemia or hypoxia, increased levels of extracellular Mg show cardioprotective effects. The mechanisms of high level Mg-induced cardioprotection were examined in Langendorff perfused rat hearts. In the control group (1.2 mM Mg during hypoxia), the recovery of the left ventricular developed pressure (LVDP) after 30 min of reoxygenation was 57.6±3.0% of the level observed before hypoxia. In the high Mg group (12 mM Mg during hypoxia), the time course of recovery was faster than in the control group; the recovery level of LVDP improved to 78.4±4.2%. This protective effect of high levels of Mg decreased to 69.0±3.6% with the application of 5-hydroxydecanoic acid (100 μM), a specific mitochondrial ATP-sensitive potassium channel (KATP) blocker. In the low Ca group (0.2 mM Ca during hypoxia), the recovery of LVDP did not reach the level observed in the high Mg group (64.7±5.9%), but with application of diazoxide, a specific mitochondrial KATP channel opener, the LVDP recovery improved to 81.8±11.1%, similar to the level observed in the high Mg group. These results suggest that cardioprotective effects of high levels of extracellular Mg during hypoxia occur not only due to energy conservation and/or by intracellular prevention of Ca2+ over-load, but also by opening of the mitochondrial KATP channel.
Keywords: Cardioprotection, Hypoxia, Magnesium, Mitochondrial KATP channel, Reperfusion
Various factors, such as the generation of reactive oxygen species (1,2), apoptosis (3–5), Ca2+ overload (6,7) and protease activation (8,9), play complicated roles in myocardial ischemia-reperfusion injury. Various methods for cardio-protection have been proposed, and among them, ischemic preconditioning, ie, repeated short-term ischemia-reperfusion cycles followed by sustained ischemia, has been reported to be effective (10–13). Although the precise mechanisms of ischemic preconditioning must still be clarified, many studies (11–13) agree that the opening of the mitochondrial ATP-sensitive potassium channel (KATP) plays an important role. However, it has also been reported that increased extracellular Mg concentrations during ischemia or hypoxia have cardioprotective effects (14–16). In fact, the concentration of Mg2+ in cardioplegic solution, which is used during cardiopulmonary bypass surgery, is 16 mM, which is more than 10-fold higher than that in plasma (14). It has been suggested that the cardioprotective effects of Mg2+ may be due to Mg2+ antagonization of Ca2+, which inhibits Ca2+ influx and Ca2+ overload. However, it is also known that during ischemia, the intracellular Mg concentration decreases (17). This suggests that when the extracellular Mg concentration is high, it prevents Mg efflux from the intracellular space, and, thus, helps maintain the intracellular environment.
We examined the mechanisms of cardioprotection caused by elevated Mg concentrations during hypoxia in Langendorff perfused rat hearts. Our results suggest that Mg protects the heart not only by energy conservation and/or by prevention of intracellular Ca2+ overload, but also by maintaining the intracellular environment, which likely led to the opening of the mitochondrial KATP channel.
MATERIALS AND METHODS
Preparation of hearts and perfusion protocols
The present investigation conformed with the Guide for the Care and Use of Laboratory Animals (18) and was approved by the Juntendo University School of Medicine (Tokyo, Japan) animal experimentation committee.
Male Sprague-Dawley rats, weighing 285 g to 360 g, were used. Preparation and perfusion protocols were almost the same as those reported previously (19). In brief, rats were decapitated, the hearts quickly excised and Langendorff perfusion was established. Each heart was perfused retrogradely with modified Krebs-Henseleit solution (containing NaCl 116.0 mM, NaHCO3 25.0 mM, CaCl2 2.5 mM, MgSO4 1.2 mM, KCl 4.7 mM, KH2PO4 1.2 mM, glucose 5.5 mM; pH 7.4) at a constant flow rate of 13 mL/min without recirculation. The perfusate was oxygenated with a 95% O2:5% CO2 gas mixture to maintain the partial pressure of O2 above 400 mmHg and was warmed to 38°C. For hypoxic perfusion, glucose was substituted by equimolar sucrose, and the perfusate was saturated with a 95% N2:5% CO2 gas mixture to maintain the partial pressure of O2 at approximately 20 mmHg. All hearts were reoxygenated with normal Krebs solution. A latex balloon was inserted through the mitral annulus into the left ventricular cavity, and distilled water (0.1 mL to 0.3 mL) was injected into the balloon until it was inflated to just above the level required to produce a visible elevation of the left ventricular end-diastolic pressure of 1 mmHg to 2 mmHg. The left ventricular developed pressure (LVDP), heart rate (HR) and coronary perfusion pressure were monitored throughout the experiments.
Experimental protocols
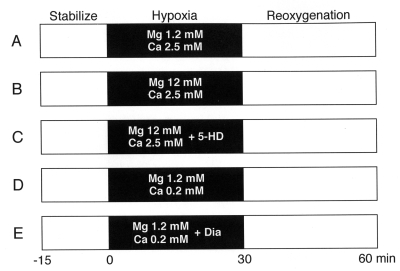
After 15 min of stabilization, hearts were exposed to 30 min of hypoxia, followed by a 30 min period of reoxgenation. The hearts were separated into five groups according to the treatments during hypoxia (Figure 1). In the control group (n=20), the hearts were made hypoxic with normal Mg (1.2 mM) and Ca (2.5 mM) concentrations (Figure 1A). In the high Mg group (n=7), during hypoxia, the concentration of Mg was increased to 12 mM (Figure 1B). In the high Mg + 5-hydroxydecanoic acid (5-HD) group (n=10), the concentration of Mg was increased to 12 mM with the addition 100 μM 5-HD, a specific mitochondrial KATP channel blocker, during hypoxia (Figure 1C). In the low Ca group (n=6), during hypoxia, the concentration of Ca was decreased to 0.2 mM without a change in the Mg concentration (Figure 1D). In the low Ca + diazoxide (Dia) group (n=6), the concentration of Ca was decreased to 0.2 mM with the addition 100 μM Dia, a specific mitochondrial KATP channel opener, during hypoxia (Figure 1E).
Figure 1).
Experimental protocols. After the 15 min stabilization period, the heart underwent hypoxia for 30 min, followed by reoxygenation for 30 min. The hearts were divided into five groups according to the hypoxic conditions. In every experiment, the concentration of Mg and Ca during stabilization (Stabilize) and reoxygenation were 1.2 mM and 2.5 mM, respectively. A Control group (n=20) was perfused with normal concentrations of Mg and Ca. B High Mg group (n=7); Mg concentration was increased to 12 mM. C High Mg + 5-hydroxydecanoic acid (5-HD) group (n=10); the Mg concentration was increased to 12 mM and 100 μM of 5-HD was added. D Low Ca group (n=6); the Ca concentration was decreased to 0.2 mM. E Low Ca + diazoxide (Dia) group (n=6); the Ca concentration was decreased to 0.2 mM and 100 μM of Dia was added
Mg assay
Tissue Mg was measured by atomic absorption spectrophotometry. At the end of the experiments, the hearts were perfused with Mg-free perfusate for 3 min, and 100 mg of organs were weighed accurately and put into a perfluoroalkoxy vial (Taf-tainer vial, GL Science, Japan). After adding 0.4 mL of concentrated nitric acid and 0.2 mL to 0.8 mL of H2O2 (Tama pure AA 110, Tama Chemicals Co Ltd, Japan), the tissue was digested in a microwave oven (MLS-1200 MEGA, Milestone SRL, Italy). The digestion program was 250 W for 5 min, 0 W for 1 min, 250 W for 5 min, 400 W for 5 min and 600 W for 5 min. The digested samples were diluted with pMilli-Q purified water (greater than 18 MΩ) up to 1.0 mL, and the appropriate dilution was performed with 0.5% nitric acid. Mg concentrations were determined using an atomic absorption spectrometer (AAnalyst 800, PerkinElmer Japan Co Ltd, Japan) with an acetylene flame. The atomic absorption spectrometry used a hollow cathode lamp as the resonance source with a lamp current of 6 mA, an analytical wavelength of 285.2 nm, background correction with Polarized-Zeeman method (20) and a slit width of 0.7 nm.
Drugs
5-HD and Dia were purchased from Sigma (USA). Both were dissolved in dimethyl sulfoxide to a concentration of 100 mM, and 1 mL of the stock solution was added to 1 L of Krebs solution. The final concentration of dimethyl sulfoxide was 0.1%, which did not affect the basal condition.
Statistical analysis
Data are expressed as mean ± SEM. Differences between means were analyzed by the unpaired Student’s t test or ANOVA and Bonferroni’s method, as deemed appropriate. P<0.05 was considered significant.
RESULTS
Effects of hypoxia-reoxygenation on heart function
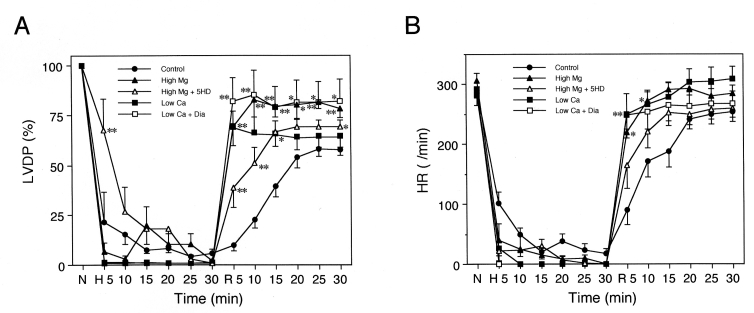
The changes in LVDP and HR by hypoxia-reoxygenation are shown in Figure 2. The level of LVDP at the end of stabilization (before hypoxia: 0 min in Figure 1) was 135.7±4.4 mmHg. In the control group, LVDP decreased to 0 mmHg during the 30 min hypoxic perfusion and recovered to 57.6±3.0% of the basal level with the 30 min of reoxygenation. In the high Mg group, LVDP decreased to 0% of the basal value within 10 min following the onset of hypoxic perfusion, and immediately recovered with reoxygenation. The recovery of LVDP after the 30 min period of reoxygenation was significantly higher than that of the control group (78.4±3.3% versus 57.6±3.0%, respectively; P<0.05). In the high Mg + 5-HD group, although the recovery of LVDP at the onset of reoxygenation was immediate, similar to that in the high Mg group, LVDP recovery after 30 min of reoxygenation was poor (69.0±3.6%; not significant versus the control group). In the low Ca group, LVDP immediately decreased to 0% with the onset of hypoxia, and recovered immediately with reoxygenation, similar to the time course in the high Mg group. However, after 5 min of reoxygenation, LVDP recovery reached a plateau, and the level was not significantly different from that of high Mg + 5-HD group (64.7±5.9% after 30 min of reoxygenation). In contrast, in the low Ca + Dia group, both the time course and the level (81.8±11.1%) of recovery were quite similar to those of the high Mg group. In every experimental group, HR recovery showed similar patterns to those of LVDP, and in the high Mg group, recovery of HR was significantly higher than that in the control group (P<0.05).
Figure 2).
Changes in left ventricular developed pressure (LVDP) (panel A) and heart rate (HR) (panel B) by hypoxia-reoxygenation. In the high Mg group (closed triangles), recovery of LVDP after 30 min reoxygenation was higher than that in the control group, and this protective effect was inhibited by application of 5-hydroxydecanoic acid (5HD) (high Mg + 5HD group; open triangles). In the low Ca group (closed squares), LVDP abruptly decreased with hypoxic perfusion and immediately recovered with reoxygenation. The level of recovery after 30 min of reoxygenation was similar to that of the high Mg + 5HD group. In the low Ca + diazoxide (Dia) group (open squares), the recovery of LVDP after 30 min of reoxygenation was better than that of the low Ca group, and reached the same level observed in the high Mg group. In the high Mg group, low Ca group and low Ca + Dia group, HR recovery after 5 min of reoxygenation was faster than that in the control group. *P<0.05 and **P<0.01 versus the control group at the same time points. H Hypoxia; N Normal
Mg content of perfused hearts
The amount of total intracellular Mg was quantified using atomic absorption spectrophotometry (Figure 3). In stabilized hearts (n=8) from the control group, the Mg concentration was 936.2±24.1 ppm/g dry weight. After 30 min of hypoxia, the Mg content of hearts decreased nonsignificantly to 883.1±20.1 ppm/g dry weight, and then decreased significantly to 750.2±24.7 ppm/g dry weight with reoxygenation (n=7; P<0.05). When the extracellular Mg concentration was increased to 12 mM during hypoxic perfusion, the Mg content of hearts at the end of hypoxia increased to 1045.7±20.1 ppm/g dry weight (n=8; P<0.05 versus control stabilized hearts). After reoxygenation, the Mg content decreased to 831.5±35.9 ppm/g dry weight (n=3), which was not statistically different from that of the control stabilized hearts.
Figure 3).
Total Mg content in hearts. Mg content in hearts was measured by atomic absorption spectrophotometry as total Mg content. The Mg content in control hearts decreased with hypoxic (Hypo) perfusion (closed column; Hypo), and further decreased with reoxygenation (light grey column; hypoxia-reoxygenation [H-R]). High Mg during hypoxia increased the Mg content (dark grey column; High Mg Hypo). Although it was decreased by reoxygenation, the level was kept at almost the same level as that of the stabilized heart (Stab) (open column). *P<0.05 and **P<0.01 versus stabilized control heart
DISCUSSION
When Mg concentrations were increased during hypoxic perfusion to 12 mM (high Mg group), a level 10-fold higher than that of normal conditions, LVDP recovery after reoxygenation improved remarkably. Two effects were observed in this improvement of LVDP. One was that the recovery of LVDP was observed immediately after the onset of reoxygenation and it increased quickly, ie, LVDP recovery at the onset of reoxygenation is fast (Figure 2A). The other effect was that the recovery level of LVDP after 30 min of reoxygenation was higher than that of the control group (78.4±3.3% versus 57.6±3.0%; Figure 2A). Because similar effects of fast LVDP recovery at the onset of reoxygenation were also observed when the extracellular Ca concentration was decreased to 0.2 mM, we can assume that energy consumption was suppressed because contraction is inhibited by high Mg or low Ca concentrations during hypoxic perfusion. However, in the high Mg group, the final level of LVDP (after 30 min of reoxygenation) was higher than that in the low Ca group. However, these results cannot be explained by the conservation of high energy phosphates, because during hypoxia, although the suppression of cardiac contractility was stronger in the low Ca group than in the high Mg group, the final LVDP level of the low Ca group was lower than that of the high Mg group.
When the extracellular Mg concentration was increased to 12 mM in the presence of 5-HD, a specific mitochondrial KATP channel blocker (high Mg + 5-HD group), the time course of LVDP recovery was faster than that of the control group and slower than that of the high Mg group, but the final LVDP level decreased to almost the same level as that of the low Ca group. Conversely, when the extracellular Ca concentration was decreased to 0.2 mM in the presence of Dia, a specific mitochondrial KATP channel opener (low Ca + Dia group), LVDP improved to a level equivalent to that of the high Mg group. These results suggest that high Mg during hypoxia confers cardioprotection which is caused not only by the conservation of high energy phosphates with Mg2+ antagonization of Ca2+, but also by a mechanism related to the opening of the mitochondrial KATP channel.
In cardiac myocytes, it is known that the intracellular total Mg concentration ([Mg]i) is approximately 10 mM, while the free Mg concentration is maintained at 0.5 mM to 1 mM (21), and changes in the extracellular Mg concentration do not affect the intracellular free Mg concentration. However, the application of isoproterenol (22,23) and ischemia (17) can induce a reduction in the intracellular free Mg concentration. For example, it was reported that myocardial total Mg decreased during ischemia, but did not decrease further with reperfusion (24). On the other hand, it has been reported that the intracellular free Mg increased during ischemia (25) or hypoxia (26). These reports suggest that the results of Mg dynamics are not straightforward. The present study showed that hypoxia also induced a reduction in the [Mg]i, which decreased further with the following reoxygenation. The reduction in [Mg]i with hypoxia was inhibited by increasing the extracellular Mg concentration during hypoxia, which also attenuated the further decrease in [Mg]i during reoxygenation. Although the cause of the decrease in [Mg]i accompanying hypoxia is unknown, it is possible that the permeability to Mg may be accelerated. Our observation that the increased extracellular Mg concentration during hypoxia increased [Mg]i to a level higher than the normal concentration may support this possibility. Our study and previous reports from other laboratories (17,24–26) suggest the possibility that changes in the distribution of intracellular free Mg influence the recovery of the heart function after ischemia and/or hypoxia. Further direct examination of intracellular free Mg dynamics using an Mg indicator, such as mag-Fura 2 and KMG-20, may be needed.
Intracellular Mg is critical to the activation of KATP channels. In the absence of Mg2+, the KATP channel does not open (27) and does not respond to a KATP channel opener (28). Moreover, MgADP strongly antagonizes the binding of ATP to the KATP channel (29), and acts as an opener itself (30). Furthermore, it has also been reported that [Mg2+]i and the activity of the membrane KATP channel are positively correlated (31). It is thought that [Mg2+]i is probably correlated with the activity of the mitochondrial KATP channel.
CONCLUSION
An increase in the extracellular Mg concentration during hypoxic perfusion inhibits the reduction of [Mg]i, resulting in increased probability of the mitochondrial KATP channel opening, leading to cardioprotection.
REFERENCES
- 1.Kuzuya T, Hoshida S, Kim Y, et al. Detection of oxygen-derived free radical generation in the canine postischemic heart during late phase of reperfusion. Circ Res. 1990;66:1160–5. doi: 10.1161/01.res.66.4.1160. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosio G, Tritto I. Reperfusion injury: Experimental evidence and clinical implications. Am Heart J. 1999;138:S69–75. doi: 10.1016/s0002-8703(99)70323-6. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Murtuza B, Smolenski RT, et al. Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation. 2001;104(Suppl I):I308–13. doi: 10.1161/hc37t1.094871. [DOI] [PubMed] [Google Scholar]
- 4.Zhao ZQ, Velez DA, Wang NP, et al. Progressively developed myocardial apoptotic cell death during late phase of reperfusion. Apoptosis. 2001;6:279–90. doi: 10.1023/a:1011335525219. [DOI] [PubMed] [Google Scholar]
- 5.Wang GW, Zhou Z, Klein JB, Kang YJ. Inhibition of hypoxia/reoxygenation-induced apoptosis in metallothionein-overexpressing cardiomyocytes. Am J Physiol Heart Circ Physiol. 2001;280:H2292–9. doi: 10.1152/ajpheart.2001.280.5.H2292. [DOI] [PubMed] [Google Scholar]
- 6.Nayler WG. The role of calcium in the ischemic myocardium. Am J Pathol. 1981;102:262–70. [PMC free article] [PubMed] [Google Scholar]
- 7.Kusuoka H, Porterfield JK, Weisman HF, Weisfeldt ML, Marban E. Pathophysiology and pathogenesis of stunned myocardium: Depressed Ca2+ activation of contraction as a consequence of reperfusion-induced cellular calcium overload in ferret hearts. J Clin Invest. 1987;79:950–61. doi: 10.1172/JCI112906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida K, Yamasaki Y, Kawashima S. Calpain activity alters in rat myocardial subfractions after ischemia or reperfusion. Biochem Biophys Acta. 1993;1182:215–20. doi: 10.1016/0925-4439(93)90143-o. [DOI] [PubMed] [Google Scholar]
- 9.Sorimachi Y, Harada K, Saido TC, Ono T, Kawashima S, Yoshida K. Downregulation of calpastatin in rat heart after brief ischemia and reperfusion. J Biochem (Tokyo) 1997;122:743–8. doi: 10.1093/oxfordjournals.jbchem.a021818. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MV, Downey JM. Myocardial preconditioning promises to be a novel approach to the treatment of ischemic heart disease. Annu Rev Med. 1996;47:21–9. doi: 10.1146/annurev.med.47.1.21. [DOI] [PubMed] [Google Scholar]
- 11.Miura T, Liu Y, Kita H, Ogawa T, Shimamoto K. Roles of mitochondrial ATP-sensitive K channels and PKC in anti-infarct tolerance afforded by adenosine A1 receptor activation. J Am Coll Cardiol. 2000;35:238–45. doi: 10.1016/s0735-1097(99)00493-3. [DOI] [PubMed] [Google Scholar]
- 12.Ohnuma Y, Miura T, Miki T, et al. Opening of mitochondrial K(ATP) channel occurs downstream of PKC-epsilon activation in the mechanism of preconditioning. Am J Physiol Heart Circ Physiol. 2002;283:H440–7. doi: 10.1152/ajpheart.00434.2001. [DOI] [PubMed] [Google Scholar]
- 13.Pain T, Yang XM, Critz SD, et al. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–6. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 14.Hearse DJ, Stewart DA, Braimbridge MV. Myocardial protection during ischemic cardiac arrest. The importance of magnesium in cardioplegic infusates. J Thorac Cardiovasc Surg. 1978;75:877–85. [PubMed] [Google Scholar]
- 15.Shakerinia T, Ali IM, Sullivan JA. Magnesium in cardioplegia: Is it necessary? Can J Surg. 1996;39:397–400. [PMC free article] [PubMed] [Google Scholar]
- 16.Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S. Protective role of magnesium in cardiovascular diseases: A review. Mol Cell Biochem. 2002;238:163–79. doi: 10.1023/a:1019998702946. [DOI] [PubMed] [Google Scholar]
- 17.Murphy E, Steenbergen C, Levy LA, Raju B, London RE. Cytosolic free magnesium levels in ischemic rat heart. J Biol Chem. 1989;264:5622–7. [PubMed] [Google Scholar]
- 18.National Institutes of Health . Guide for the Care and Use of Laboratory Animals (Publication no 85-23) Washington: National Academy Press; 1985. [Google Scholar]
- 19.Watanabe M, Okada T. Lysophosphatidylcholine-induced myocardial damage is inhibited by pretreatment with poloxamer 188 in isolated rat heart. Mol Cell Biochem. 2003;248:209–15. doi: 10.1023/a:1024165125139. [DOI] [PubMed] [Google Scholar]
- 20.Pleban PA, Kerkay J, Pearson KH. Polarized Zeeman-effect flameless atomic absorption spectrometry of cadmium, copper, lead, and manganese in human kidney cortex. Clin Chem. 1981;27:68–72. [PubMed] [Google Scholar]
- 21.Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe J, Nakayama S, Matsubara T, Hotta N. Regulation of intracellular free Mg2+ concentration in isolated rat hearts via beta-adrenergic and muscarinic receptor. J Mol Cell Cardiol. 1998;30:2307–18. doi: 10.1006/jmcc.1998.0791. [DOI] [PubMed] [Google Scholar]
- 23.Romani AM, Scarpa A. Regulation of cellular magnesium. Front Biosci. 2000;5:D720–34. doi: 10.2741/romani. [DOI] [PubMed] [Google Scholar]
- 24.Tosaki A, Szerdahelyi P, Engelman RM, Das DK. Effects of extracellular magnesium manipulation on reperfusion-induced arrhythmias and myocardial ion shifts in isolate ischemic reperfused rat hearts. J Pharmacol Exp Ther. 1993;267:1045–53. [PubMed] [Google Scholar]
- 25.Murphy E, Steenbergen C, Levy LA, Raju B, London RE. Cytosolic free magnesium levels in ischemic rat heart. J Biol Chem. 1989;264:5622–7. [PubMed] [Google Scholar]
- 26.Silverman HS, Lisa FD, Hui RC, et al. Regulation of intracellular free Mg2+ and contraction in single adult mammalian cardiac myocytes. Am J Physiol. 1994;266:C222–33. doi: 10.1152/ajpcell.1994.266.1.C222. [DOI] [PubMed] [Google Scholar]
- 27.Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993;110:573–82. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards G, Ibbotson T, Weston AH. Levcromakalim may induce a voltage-independent K-current in rat portal veins by modifying the gating properties of the delayed rectifier. Br J Pharmacol. 1993;110:1037–48. doi: 10.1111/j.1476-5381.1993.tb13918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueda K, Inagaki N, Seino S. MgADP antagonism to Mg2+-independent ATP binding of the sulfonylurea receptor SUR1. J Biol Chem. 1997;272:22983–6. doi: 10.1074/jbc.272.37.22983. [DOI] [PubMed] [Google Scholar]
- 30.Shyng S, Ferrigni T, Nichols CG. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J Gen Physiol. 1997;110:643–54. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leyssens A, Nowicky AV, Patterson L, Crompton M, Duchen MR. The relationship between mitochondrial state, ATP hydrolysis, [Mg2+]i and [Ca2+]i studied in isolated rat cardiomyocytes. J Physiol. 1996;496:111–28. doi: 10.1113/jphysiol.1996.sp021669. [DOI] [PMC free article] [PubMed] [Google Scholar]