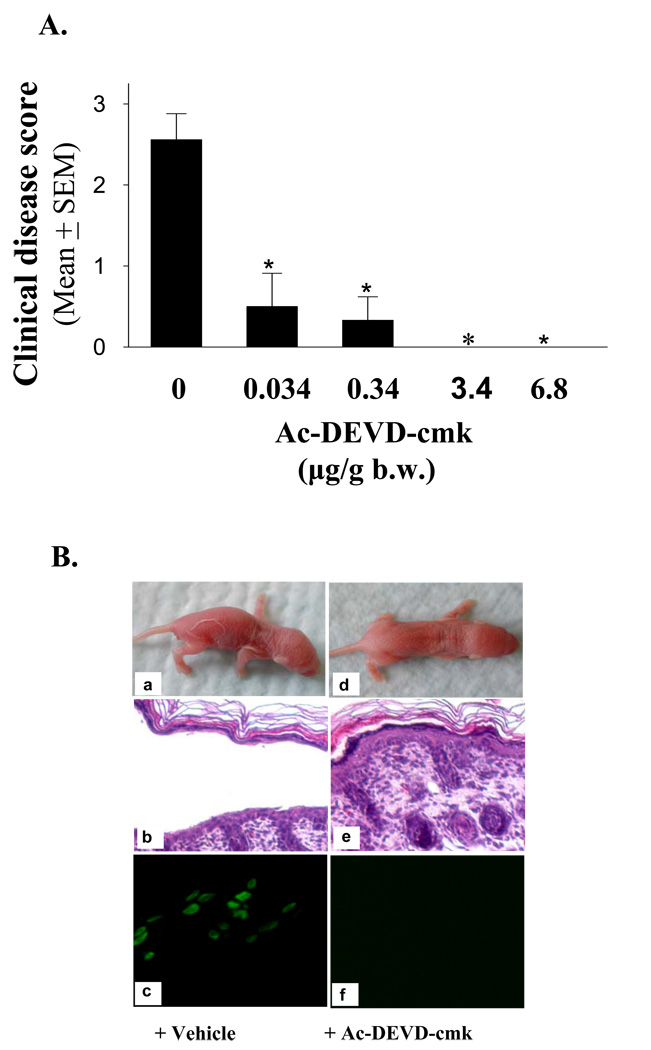
Figure 4. The caspase-3/7 inhibitor Ac-DEVD-cmk protects mice against PF.
(A) Dose-dependent protective effects. Various doses of caspase-3/7 inhibitor Ac-DEVD-cmk were administrated into neonatal mice by two subcutaneous injections. Half of the dose was given 1.5 h before IgG injection, and the second half dose was injected concomitantly with PF1 IgG. Control mice were injected with vehicle. The same dose of pathogenic PF1 IgG was used for disease induction in each experiment. The extent of disease was examined and scored 20 h post IgG injection. The protective effect is highly significant (n=8 for the control group, n=3 for each treatment group, *p<0.001, Student t-test). (B) Suppression of TUNEL-staining and blockade of intraepidermal blisters and clinical disease. Neonatal mice were injected with pathogenic PF1 IgG with (d–f) or without (a–c) Ac-DEVD-cmk. Control mice treated with vehicle developed clinical and histological blisters (a and c) 20 hours post IgG injection. In contrast, mice treated with the caspase-3 inhibitor did not develop clinical and histological lesions (d and f). TUNEL assay showed a positive staining in control mice but not in mice treated with the inhibitor (e).