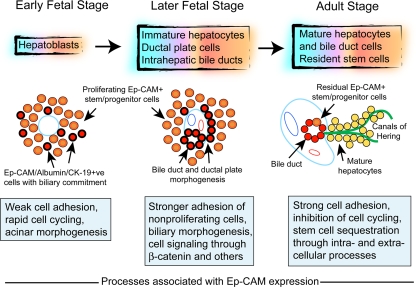
Fig. 10.
Schematic representation of changes during liver development. In this scheme, hepatoblasts in the fetal liver parenchyma originate from primitive endoderm and give rise to immature hepatocytes, ductal plate cells, and intrahepatic ducts, which in turn generate mature hepatocytes and biliary cells. The cartoons depict morphogenetic changes during early fetal stage (left), for example, 7 weeks, where the liver contains loosely arranged Ep-CAM+ stem/progenitor cells (cells in pink with black and red borders indicating coexpression of E-cadherin and Ep-CAM, respectively), while at later fetal stages (middle), for example, after 12 weeks, biliary morphogenesis leads to ductal plate cells and onset of biliary morphogenesis with high-level expression of Ep-CAM and CK-19 along with E-cadherin (cells shown in red with black borders). The adult stage (right) is characterized by the familiar hepatic and biliary compartments, although residual fetal-type stem/progenitor cells remain in ductular structures with likely access to the parenchyma through canals of Hering. The boxes indicate that during the early fetal stage, weak cell adhesion through focally expressed Ep-CAM would promote cell proliferation and acinar organization. During later fetal stages (middle), extensive Ep-CAM expression and altered intracellular β-catenin activity, along with other regulatory factors, would impede cell proliferation, facilitate biliary morphogenesis and help sequester stem/progenitor cells in the ductal plate, and primitive bile ducts. In the adult liver (right), strong E-cadherin-mediated adhesion in the absence of Ep-CAM would inhibit proliferation-initiating events in mature hepatocytes, while Ep-CAM will help sequester fetal-type stem/progenitor cells in ductal niches, largely in a quiescent stage, until these cells must replenish lost hepatocytes