Figure 4.
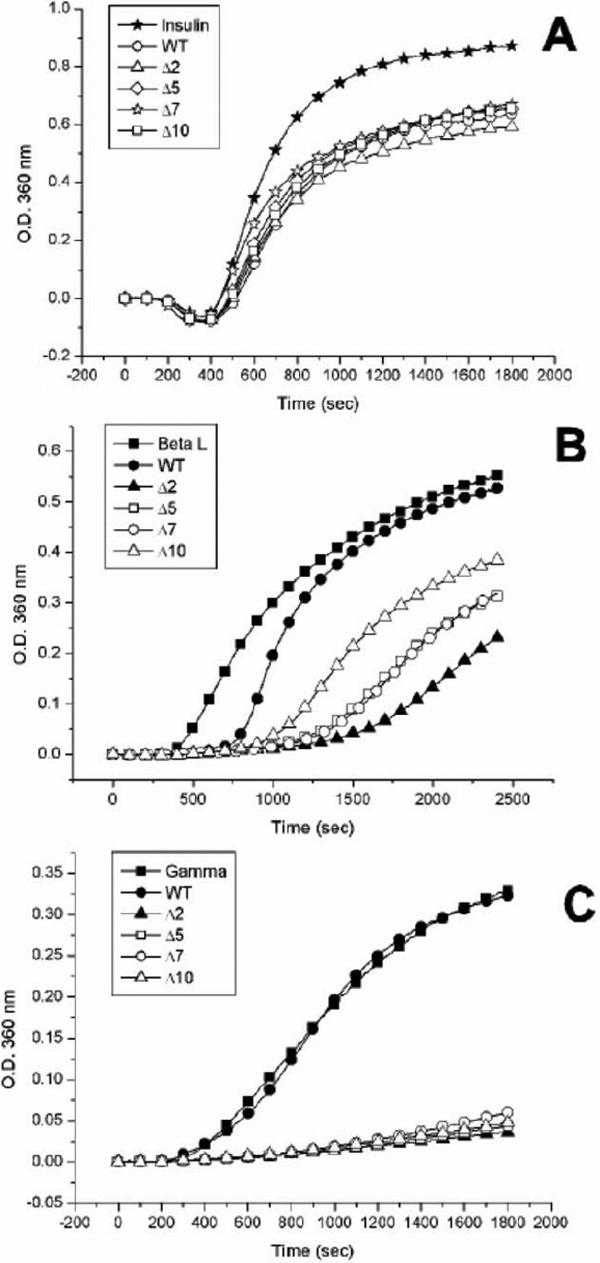
Comparison of chaperone activities of various αB-crystallin and deletion mutants against chemical and thermal denaturation. A: Inhibition of DTT-induced insulin B chain aggregation by wild-type αB-crystallin and its truncated mutants. The mutant proteins (Δ2, Δ5, Δ7, and Δ10) all show similar chaperone activities with wild-type αB-crystallin at 37 °C in a molar ratio (chaperone/insulin) of 1:16. The final concentration of bovine pancreas insulin is 59.3 μM. B: Inhibition of thermal denaturation of porcine βL-crystallin by wild-type αB-crystallin and its truncated mutants. βL-crystallin was used as a substrate for chaperone-activity assays at 60 °C. Mutant proteins (Δ2, Δ5, Δ7, and Δ10) all show higher chaperone activity than wild-type αB-crystallin in a molar ratio of (chaperone/βL-crystallin) of 2:7, with Δ2 showing highest activity among 4 deletion mutants. The final concentration of porcine βL-crystallin is 6.2 μM. C: Chaperone-activity assays using porcine γ-crystallin as a substrate. The wild-type αB-crystallin and its truncated mutants were heated at 65 °C in a molar ratio of 1:1 (chaperone/γ-crystallin). The mutant proteins (Δ2, Δ5, Δ7, and Δ10) all show better chaperone activities than wild-type αB-crystallin which exhibits no chaperone activity for heat denaturation of γ-crystallin. The final concentration of porcine γ-crystallin is 2.6 μM.
