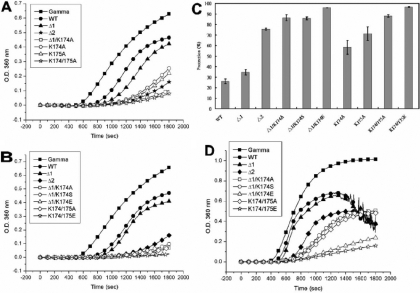
Figure 7.
Comparison of chaperone activities of wild-type αB-crystallin and its mutants under thermal denaturation. A: Porcine α-crystallin was used as a substrate for chaperone-activity assays of wild-type αB-crystallin and mutants at 65 °C. Mutant proteins and wild-type αB-crystallin showed different chaperone activities at a molar ratio of 2:3 (chaperone/γ-crystallin). The scattering curves at 360 nm in the presence of chaperoning crystallins are shown as follows: control solution without chaperone (closed square), wild-type αB-crystallin (closed circle), Δ1 (closed triangle), Δ2 (closed asterisk), Δ1/K174A (open square), K174A (open circle), K175A (open triangle), and K174/175A (open asterisk). It is noted that K174/175A and Δ1/K174A show the highest activity among all mutants. B: Comparison of chaperone activities of wild-type αB-crystallin and mutants with different electrostatic amino acids under identical conditions as in A. The scattering curves at 360 nm in the presence of chaperoning crystallins are shown as follows: control solution without chaperone (closed square), wild-type αB-crystallin (closed circle), Δ1 (closed triangle), Δ2 (closed rhombus), Δ1/K174A (open square), Δ1/K174S (open circle), Δ1/K174E (open triangle), K174/175A (open rhombus), and K174/175E (open asterisk). C: Comparison of chaperone activity (percentage protection) of wild-type αB-crystallin and mutants. Wild-type αB-crystallin was shown to possess poor protective activity and K174/175E shown to possess the best protective activity among all proteins. The final concentration of porcine α-crystallin is 5.5 μM. D: Chaperone activities of wild-type αB-crystallin and its mutants under thermal denaturation at 70 °C. Porcine α-crystallin was used as a substrate for chaperone-activity assays of wild-type αB-crystallin and its mutants with different electrostatic amino acids at 70 °C in a molar ratio of 2:3 (chaperone/γ-crystallin). The scattering curves at 360 nm in the presence of chaperoning crystallins are shown as follows: control solution without chaperone (closed square), wild-type αB-crystallin (closed circle), Δ1 (closed triangle), Δ2 (closed rhombus), Δ1/K174A (open square), Δ1/K174S (open circle), Δ1/K174E (open triangle), K174/175A (open rhombus), and K174/175E (open asterisk). Both Δ1/K174E and K174/175E show the best protective activity among all mutants under these conditions. The final concentration of porcine α-crystallin is 5.5 μM.