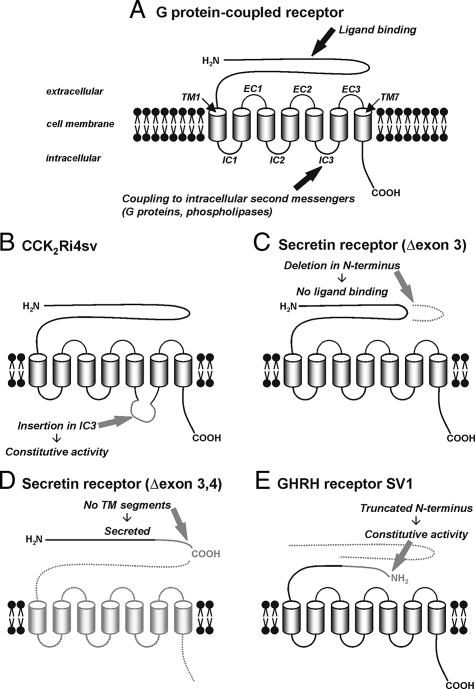
Figure 3.
Alternative splicing of G protein-coupled peptide hormone receptors. A: Schematic structure of G protein-coupled peptide hormone receptors with the functionally important domains. The extracellular N-terminal domain provides the ligand binding site. The seven transmembrane segments (TM) anchor the receptor in the cell membrane; they are connected by three intracellular (IC) and three extracellular (EC) loops. The intracellular loops, especially the third, couple to intracellular second messengers as a result of a conformational change of the receptor on ligand binding and are, thus, important for intracellular signal transduction. The intracellular C-terminal domain mediates receptor desensitization and internalization. B–E: Typical structural changes in receptor splice variants. B: An extension in the third intracellular loop of the CCK2 receptor, created by intron 4 retention, is associated with constitutive activation of intracellular signaling pathways in the absence of a ligand. C: An in-frame deletion within the extracellular N-terminal domain of the secretin receptor, originating from exon 3 skipping, inhibits ligand binding. D: A deletion in the N-terminal domain of the secretin receptor produced by skipping of exons 3 and 4 leads to a frame-shift, early stop codon, and truncation; in the absence of any transmembrane domain, the receptor is not anchored in the cell membrane and secreted from the cell. E: N-terminal truncation of the GHRH receptor in SV1, created by deletion of exons 1 through 3 and retention of intron 3, leads to receptor activity in the absence of ligand binding.