Abstract
Immune cells are critical to the wound-healing process, through both cytokine and growth factor secretion. Although previous studies have revealed that B cells are present within wound tissue, little is known about the role of B cells in wound healing. To clarify this, we investigated cutaneous wound healing in mice either lacking or overexpressing CD19, a critical positive-response regulator of B cells. CD19 deficiency inhibited wound healing, infiltration of neutrophils and macrophages, and cytokine expression, including basic and acidic fibroblast growth factor, interleukin-6, platelet-derived growth factor, and transforming growth factor-β. By contrast, CD19 overexpression enhanced wound healing and cytokine expression. Hyaluronan (HA), an endogenous ligand for toll-like receptor (TLR)-4, stimulated B cells, which infiltrates into wounds to produce interleukin-6 and transforming growth factor-β through TLR4 in a CD19-dependent manner. CD19 expression regulated TLR4 signaling through p38 activation. HA accumulation was increased in injured skin tissue relative to normal skin, and exogenous application of HA promoted wound repair in wild-type but not CD19-deficient mice, suggesting that the beneficial effects of HA to the wound-healing process are CD19-dependent. Collectively, these results suggest that increased HA accumulation in injured skin induces cytokine production by stimulating B cells through TLR4 in a CD19-dependent manner. Thus, this study is the first to reveal a critical role of B cells and novel mechanisms in wound healing.
Healing of cutaneous wounds is a complex biological event that results from the interplay of a large number of resident and infiltrating cell types, including leukocytes.1 Accumulating evidence has revealed a critical role of leukocytes in wound healing. Infiltrating neutrophils form a first line of defense against local infections and are also a source of pro-inflammatory cytokines to activate fibroblasts and keratinocytes.2 Macrophages also regulate wound healing by antimicrobial function, wound debridement, and production of various growth factors, such as platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β, basic fibroblast growth factor (bFGF), heparin binding epidermal growth factor, and TGF-α.3,4,5,6 These factors stimulate the synthesis of extracellular matrix by local fibroblasts, generate new blood vessels, promote the granulation tissue formation, and enhance re-epithelialization.4,5 Furthermore, a series of experimental studies have indicated a significant role for T lymphocytes in wound healing as growth factor-producing cells as well as immunological effector cells.1,7,8,9 Thus, immune cells have an integral function in wound healing beyond their role in inflammation and host defense, mainly through the secretion of signaling molecules, such as cytokines and growth factors.6
However, little is known regarding a role of B cells in wound healing. Previous studies have revealed that B cells are present within wound tissue9,10,11 and that B cell count is rapidly increased in the first 4 days after wounding, and thereafter reaches a plateau before falling as the wounds heal.11 Furthermore, recent assessment of the role of B cells in the immune system has indicated that B cells are more than just the precursors of antibody (Ab)-secreting cells.12 B cells have essential functions in regulating immune responses, including the production of various cytokines and growth factors, antigen presentation, regulation of T cell activation and differentiation, and regulation of lymphoid organization.12 Therefore, the increased numbers of B cells within wound tissue may reflect a role for these cells that has hitherto been unrecognized.
Both innate and adaptive immune responses contribute to host defense cooperatively. B cells play a principal role in adaptive immune response through B cell antigen receptor (BCR). BCR-induced signals are further modified by other cell surface molecules including CD19. CD19, a major positive response regulator, is a critical B cell-specific signal transduction molecule of the immunoglobulin superfamily expressed by early pre-B cells from the time of heavy chain rearrangement until plasma cell differentiation.13 B cells also primarily participate in innate immunity; indeed, B cells express toll-like receptors (TLRs) and respond to exogenous innate stimuli such as lipopolysaccharide (LPS), a major component of Gram-negative bacteria. CD19 also regulates LPS signaling: B cells from CD19-deficient (CD19−/−) mice are hyporesponsive to most transmembrane signals, including BCR ligation and LPS, while B cells from CD19-transgenic (CD19Tg) mice that overexpress CD19 by ∼threefold are hyperresponsive to these signals.14,15 Thus, CD19 regulates both adaptive and innate immune responses.
In the current study, to clarify the roles of B cells in wound healing, we investigated the wound-healing model in CD19−/− and CD19Tg mice. The results of this study indicate that CD19 controls cytokine and growth factor production by B cells mainly through TLR4 signaling, which was activated by an endogenous TLR4 ligand hyaluronan (HA) increased in the wounded skin, and thereby CD19 regulates the skin wound-healing process.
Materials and Methods
Mice
CD19−/− (C57BL/6 × 129) and CD19Tg (C57BL/6 × B6/SJL) mice were generated as described.14,16 All mice were healthy, fertile, and did not display any evidence of infection or disease. All mice were backcrossed between 5 to 10 generations onto the C57BL/6 background. Mice were 7 to 12 weeks old for all experiments and age-matched wild-type littermates or C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were used as controls. All mice were housed in a specific pathogen-free barrier facility and screened regularly for pathogens. All studies and procedures were approved by the Committee on Animal Experimentation of Nagasaki University Graduate School of Biomedical Sciences.
Wounding and Macroscopic Examination
Mice were anesthetized with diethyl ether and their backs were shaved and wiped with 70% alcohol. Four full-thickness excisional wounds per mouse were made using a disposable sterile 6-mm biopsy punch (Maruho, Osaka, Japan), as described elsewhere.17 After wounds were covered with an occlusive dressing (Tegaderm, 3M Canada, London, ON), mice were caged individually. At 3 and 7 days after wounding, mice were anesthetized, and areas of open wounds were measured by tracing the wound openings onto a transparency. Any signs suggestive for local infection were not detected in the wounded skin. All four wounds were analyzed for macroscopic analysis of wound closure; however, the wound that was extremely distorted and was difficult to evaluate the correct size was excluded. For macroscopic analysis of wound closure, 50 wounds from 15 mice were used in wild-type group, and 42 wounds from 14 mice were used in CD19−/− and CD19Tg group.
Histological Assessment of Wound Healing
After the mice were sacrificed, wounds were harvested with a 2-mm rim of unwounded skin tissue. The wounds were fixed in 3.5% paraformaldehyde and was then paraffin embedded. Six-μm paraffin sections were stained with H&E. The epithelial gap, which represents distance between the leading edge of migrating keratinocytes, was measured under a light microscope. We identified the area that consists of newly formed capillaries and the collection of fibroblasts and macrophages as granulation tissue. Wound sections were visualized in the color monitor (PVM-14M4J, Olympus, Tokyo, Japan) using the CCD camera (CS-900, Olympus). Then, the area of granulation tissue was gated and measured by the video micrometer (VM-60, Olympus). The number of neutrophils and mast cells was counted in the entire section outside the blood vessels by H&E staining and toluidine blue staining. All of the sections were examined independently by two investigators in a blinded fashion.
Immunohistochemical Staining
Paraffin-embedded tissues were cut into 6 μm sections, deparaffinized in xylene, and rehydrated in PBS. Deparaffinized sections were treated with endogenous peroxidase blocking reagent (DAKO Cytomation A/S, Copenhagen, Denmark) and proteinase K (DAKO Cytomation A/S) for 6 minutes at room temperature. Sections were then incubated with rat monoclonal Ab (mAb) specific for macrophages (F4/80; American Type Culture Collection, Rockville, MD), CD3 (Dainippon Pharmaceutical Company, Osaka, Japan), and B220 (BD PharMingen, San Diego, CA). Rat IgG (Southern Biotechnology Associates Inc., Birmingham, AL) was used as a control for nonspecific staining. Sections were sequentially incubated with a biotinylated rabbit anti-rat IgG secondary Ab (Vectastain ABC method, Vector Laboratories, Burlingame, CA), then horseradish peroxidase-conjugated avidin-biotin complexes. Sections were washed three times with PBS between incubations. Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride and hydrogen peroxide, and then counterstained with methyl green. Stained cells were counted in nine high-power fields (0.07 mm2, magnification, ×400) in the wound bed per section. Among the nine fields, six fields were selected from both edges of the wound bed, and the remaining three fields were chosen from the middle of the wound bed. Each section was examined independently by two investigators in a blinded fashion.
For HA staining, the deparaffinized sections were incubated with 1.5% hydrogen peroxide for 10 minutes to block tissue peroxidase activity. Thereafter, sections were incubated overnight at 4°C with 5 μg/ml of biotinylated HA binding protein (Sigma-Aldrich Co., St. Louis, MO), followed by incubation (30 minutes, 37°C) with streptavidin-horseradish peroxidase (BD PharMingen). Sections were developed and counterstained as described above.
For double staining of B cells, the sections were incubated overnight at 4°C with rat anti-mouse B220 Ab (R&D systems, Minneapolis, MN), then sequentially incubated with a biotinylated rabbit anti-rat IgG secondary Ab and horseradish peroxidase-conjugated avidin-biotin complexes (Vectastain Elite ABC kit, Vector Laboratories). Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride and hydrogen peroxide. Thereafter, sections were incubated overnight at 4°C with goat anti-mouse interleukin (IL)110 Ab (Santa Cruz Biotechnology, Santa Cruz, CA), then sequentially incubated with an alkaline phosphatase-conjugated rabbit anti-goat IgG secondary Ab (Vectastain Elite ABC-AP kit, Vector Laboratories). Sections were developed with Vector Blue.
RNA Isolation and Real-Time PCR
Total RNAs were extracted from wounded skin samples using Qiagen RNeasy spin columns (QIAGEN, Crawley, UK) and subsequently were reverse transcribed to cDNA using Ready-To-Go RT-PCR Beads (GE Health care, Buckinghamshire, UK). Expression of bFGF, acidic FGF (aFGF), IL-6, IL-10, TGF-β1, PDGF, interferon (IFN)-γ, tumor necrosis factor (TNF)- α was quantified by real-time PCR using sequence-specific primers and probes designed by predeveloped TaqMan assay reagents (40 cycles of denaturing at 92°C for 15 seconds and annealing at 60°C for 60 seconds) and an ABI Prism 7300 Sequence Detector (Applied Biosystems, Foster City, CA). Glyceraldehyde-3-phosphate was used to normalize mRNA. Relative expression of real-time PCR products was determined by using the ΔΔCt method18 to compare target gene and housekeeping gene mRNA expression. One of the control samples was chosen as a calibrator sample.
B Cell Purification and Stimulation
Splenic B cells were purified (>95% B220+) by removing T cells with anti-Thy1.2 Ab-coated magnetic beads (Dynal, Lake Success, NY), macrophage/monocytes, dendritic cells, and NK cells with rat anti-CD43 mAb and sheep anti-rat IgG-coated magnetic beads (Dynal). Purified splenic B cells were cultured in 0.6 ml of culture medium in 48-well flat-bottom plates and stimulated with 10 or 100 μg/ml of LPS (0111:B4; Sigma-Aldrich). In other experiments, B cells were cultured with 200 or 500 μg/ml of HA (MP Biomedicals, Solon, OH), 1000 μg/ml of fibrinogen (Sigma-Aldrich), 100 μg/ml of HS (Sigma-Aldrich), 200 μg/ml of dermatan sulfate (Sigma-Aldrich), or 200 μg/ml of chondroitin sulfate (Sigma-Aldrich) for 12 hours. Anti-mouse TLR4 mAb (MTS510; BioLegend, San Diego, CA) or control rat IgG2a (R&D systems) was added 60 minutes before HA stimulation at concentrations of 100 μg/ml. In the experiments reported here, culture medium contained <1 U/ml endotoxin determined by Limulus amebocyte lysate assay (Associates of Cape Cod, Falmouth, MA), and supplementation of HA, fibrinogen, HS, dermatan sulfate, and chondroitin sulfate did not significantly increase endotoxin levels. In addition, all treatments with HA, fibrinogen, HS, dermatan sulfate, and chondroitin sulfate were in the presence of 10 μg/ml of polymyxin B (Calbiochem, Darmstadt, Germany) to exclude the effects of any contaminating LPS on experimental conditions. Expression of IL-6, IL-10, TGF-β1, and TNF-α was analyzed using a real-time PCR quantification method. Culture supernatants from unstimulated or stimulated B cells were also analyzed for the production of these cytokines by specific enzyme-linked immunosorbent assay (ELISA) kits (IL-6 and IL-10; Invitrogen Corp., Carlsbad, CA, and TGF-β1; Biosource International Inc., Camarillo, CA, and TNF-α; Bender MedSystems Inc., Burlingame, CA).
Macrophage Purification and Stimulation
Macrophages were also purified from single cell splenocyte suspensions by using rat IgG anti-F4/80 mAb (MCAP 497; Serotec Ltd, Oxford, UK) and sheep anti-rat IgG-coated magnetic beads (Dynal) as described elsewhere.19,20 Flow cytometric analysis confirmed the adequacy of the separation (>95%). Purified macrophages were cultured in 0.6 ml of serum-free RPMI medium in 48-well flat-bottom plates at 37°C for 8 hours with IL-6 (Acris antibodies, Hiddenhausen, Germany), IL-10 (Acris antibodies), or TGF-β1 (R&D systems) at the indicated concentrations. Expression of bFGF was analyzed using a real-time PCR quantification method. Culture supernatants from unstimulated or stimulated macrophages were also analyzed for the production of bFGF by specific ELISA kit (R&D systems).
Proliferation Assay of Keratinocytes and Fibroblasts
Primary murine keratinocytes and fibroblasts were isolated from wild-type, CD19−/−, and CD19Tg mice as previously described.21 Cells (4 × 103 cells) were cultured in 0.2 ml of Dulbecco’s modified eagle medium in 96-well culture plates with or without 1 ng/ml of bFGF (Kaken Pharmaceutical, Tokyo, Japan) for 72 hours. Cellular proliferation was quantified by the addition of 10 μmol/L 5-bromo-2-deoxyuridine (BrdU; Roche Diagnostics, Mannheim, Germany) during the last 24 hours of a 72-hour culture, and BrdU incorporation was assayed by ELISA (Roche Diagnostics), according to the manufacturer’s instructions.
In Vitro p38 Mitogen-Activated Protein Kinase Assay
Purified splenic B cells (1 × 107 cells) from wild-type, CD19−/−, and CD19Tg were stimulated with 10 μg/ml of LPS (Sigma-Aldrich), and subsequently lysed for 30 minutes on ice in buffer containing 100 mmol/L Tris (pH7.4), 100 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L NaF, 20 mmol/L Na4P2O7, 2 mmol/L Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 1 mmol/L PMSF, and protease inhibitors. Protein concentrations were determined by light absorbance at 280 nm. The phosphorylation level of p38 was analyzed using PhosphoDetect p38 mitogen-activated protein kinase (MAPK) (pThr180/pTyr182) ELISA kit (Calbiochem).
Topical Application of Growth Factors and HA
Growth factors (bFGF and PDGF) or HA were applied to each wound in 20-μl aqueous buffer immediately and 12 hours after wounding, and wounds were covered with an occlusive dressing (Tegaderm, 3M Canada). The amounts of growth factors or HA used in this study were as follows: bFGF (Kaken Pharmaceutical), 1000 ng/20 μl; PDGF B-B isoform (AUSTRAL Biologicals, San Ramon, CA), 800 ng/20 μl; and HA (MP Biomedicals), 20 μg/20 μl. Optimal amounts of growth factors were determined elsewhere.22 Macroscopic area of the open wound was measured at 3 and 7 days after wounding. For the analysis, 15 mice (50 wounds) in wild-type group, 14 mice (42 wounds) in CD19−/−, and CD19Tg group, and 10 mice (38 wounds) in groups of CD19−/− treated with HA or growth factors were used.
Statistical Analysis
The Mann-Whitney U-test was used for determining the level of significance of differences between samples, and Bonferroni’s test was used for multiple comparisons. A P value <0.05 was considered statistically significant.
Results
Macroscopic Wound Healing, Re-Epithelialization, and Granulation Tissue Formation
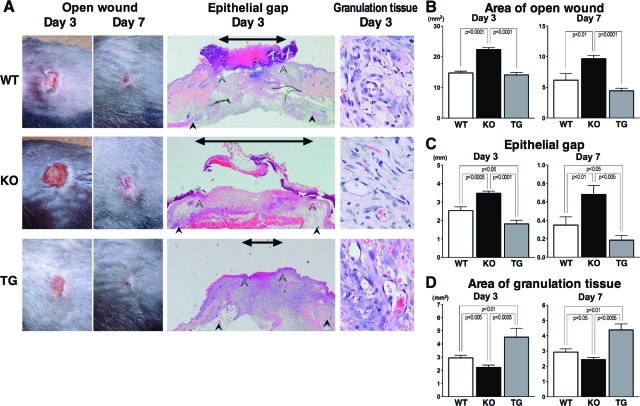
The areas of open wounds were measured at 3 and 7 days after wounding to assess macroscopic healing defects (Figure 1, A and B). At both 3 and 7 days after injury, open wound area was larger in CD19−/− mice than that in wild-type and CD19Tg mice. However, macroscopic wound healing in CD19Tg mice was similar to that in wild-type mice. Re-epithelialization was assessed by microscopically measuring the epithelial gap that is the distance between the migrating edges of keratinocytes (Figure 1, A and C). The epithelial gap was longer in CD19−/− mice relative to wild-type mice, at both 3 and 7 days after wounding. Inversely, it was shorter in CD19Tg mice compared with wild-type mice. The area of granulation tissue was also measured microscopically (Figure 1, A and D). Granulation tissue formation was inhibited in CD19−/− mice relative to wild-type and CD19Tg mice at both 3 and 7 days after wounding. Inversely, CD19 overexpression promoted granulation tissue formation relative to wild-type mice. Thus, CD19 loss inhibited cutaneous wound healing, whereas CD19 overexpression promoted it.
Figure 1.
Wound closure and granulation tissue formation in wild-type (WT), CD19−/− (KO), and CD19Tg (TG) mice at 3 and 7 days after wounding. A: Representative photographs of open wounds and histology of wound tissues (epithelial gap and granulation tissue) in wild-type, CD19−/−, and CD19Tg mice (magnification = original ×40 and × 400). In histological sections, the edges of the epithelium and panniculus carnosus are indicated by open and filled arrowheads, respectively. The epithelial gap, which is the distance between edges of the epithelium, is indicated by an arrow. B: The area of open wound was measured at 3 and 7 days after wounding by tracing of the wound openings onto a transparency. The epithelial gap (C) and the area of granulation tissue (D) were both microscopically measured in the tissue sections. Each histogram shows the mean ± SEM values obtained from 15 mice (50 wounds) in the wild-type group, and 14 mice (42 wounds) in the CD19−/− and CD19Tg groups.
Infiltration of Inflammatory Cells
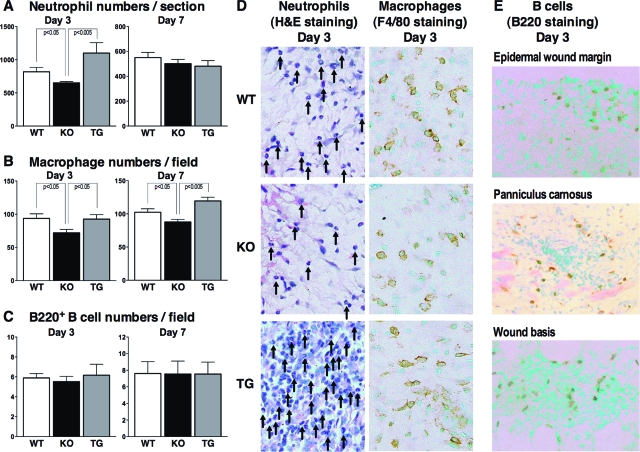
The number of neutrophils that migrated outside the blood vessels was reduced in CD19−/− mice relative to wild-type mice at 3 days but not 7 days after wounding (Figure 2, A and D). Neutrophil numbers in CD19Tg mice tended to be higher than those found in wild-type mice after 3 days; however, the difference did not reach statistical significance (P = 0.09). Macrophage numbers decreased in CD19−/− mice relative to wild-type mice as well as CD19Tg mice at both 3 and 7 days after injury (Figure 2, B and D). However, CD19 overexpression did not affect macrophage numbers. By contrast, there were no significant differences in numbers of mast cells, CD3+ T cells, and B220+ B cells among the different genotypes of mice at both 3 and 7 days after injury (Figure 2C and data not shown). However, infiltration of B cells in the wound tissue was confirmed (Figure 2E). There was no significant difference in B cell numbers among different wound sites (epidermal wound margin, edges of panniculus carnosus, and wound basis; Figure 2E and data not shown). The number of resident cutaneous inflammatory cells at the time of pre-wounding (day 0) did not differ significantly among the different genotypes (data not shown). Thus, CD19 deficiency reduced infiltration of neutrophils and macrophages, whereas altered CD19 expression did not affect the infiltration of mast cells, CD3+ T cells, or B220+ B cells.
Figure 2.
Inflammatory cell recruitment in wounded skin from wild-type (WT), CD19−/− (KO), and CD19Tg (TG) mice at 3 and 7 days after injury. A: Numbers of neutrophils per section were determined by counting H&E-stained sections under the microscope. Numbers of F4/80+ macrophages (B) and B220+ B cells (C) per field (0.07 mm2) were also counted under the microscope. D: Representative histological skin sections from wild-type, CD19−/−, and CD19Tg mice at 3 days after wounding (original magnification, ×400). Arrows represent neutrophils. E: Representative histological skin sections from wild-type mice at 3 days after wounding (magnification = original ×400). Each histogram shows the mean ± SEM values obtained from 10 mice (10 wounds) in each group.
Cytokine mRNA Expression in Wounded Skin Tissue
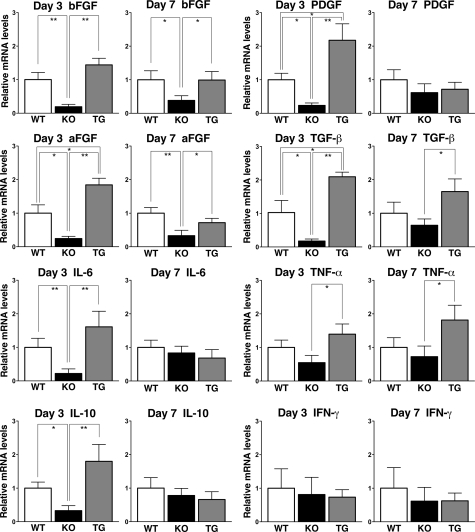
Expression of bFGF, aFGF, IL-6, IL-10, TGF-β, PDGF, TNF-α, and IFN-γ mRNA in wounded skin tissue was examined by real-time PCR (Figure 3). At 3 days after wounding, CD19−/− mice had decreased mRNA expression levels of bFGF, aFGF, IL-6, IL-10, PDGF, and TGF-β relative to wild-type mice and CD19Tg mice, whereas at 7 days after wounding, the significant difference between CD19−/− and wild-type mice was observed only in bFGF and aFGF mRNA levels. By contrast, CD19Tg mice had elevated levels of aFGF, PDGF, and TGF-β mRNA relative to wild-type mice and CD19−/− mice at 3 days after wounding. Although levels of bFGF, IL-6, IL-10, and TNF-α in CD19Tg mice were higher than those in CD19−/− and were also tend to be higher compared with wild-type mice, these differences did not reach statistical significance. At 7 days after wounding, bFGF, aFGF, TGF-β, and TNF-α mRNA levels were elevated in CD19Tg mice relative to CD19−/− mice, while they were similar between CD19Tg and wild-type mice. Finally, the loss or overexpression of CD19 did not affect IFN-γ mRNA expression. Thus, CD19 deficiency reduced expression of bFGF, aFGF, IL-6, IL-10, PDGF, and TGF-β, while CD19 overexpression increased expression of aFGF, PDGF, and TGF-β.
Figure 3.
Cytokine production in the wounded skin tissue of wild-type (WT), CD19−/− (KO), and CD19Tg (TG) mice. Relative mRNA expression of bFGF, aFGF, IL-6, IL-10, PDGF, TGF-β, TNF-α, and IFN-γ was quantified by real-time PCR and normalized relative to endogenous glyceraldehyde-3-phosphate dehydrogenase levels. Each histogram shows the mean ± SEM values obtained from 10 mice (10 wounds) in each group. *P < 0.05, **P < 0.01.
Cytokine Expression by B Cells Stimulated with LPS
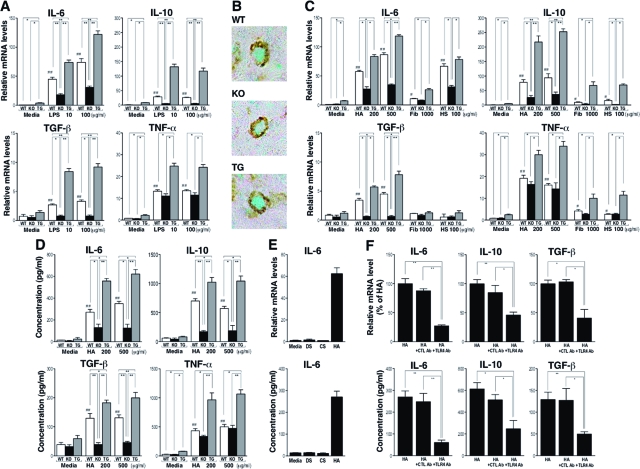
Since CD19 regulates B cell response by LPS stimulation,14,15 it is possible that CD19 affects TLR4-mediated cytokine production by B cells, resulting in the different cytokine levels within the wound tissue among the different genotypes. Therefore, we stimulated B cells with LPS and examined their cytokine mRNA expression by real-time PCR analysis (Figure 4A). Unstimulated B cells from CD19Tg mice spontaneously produced a higher mRNA amount of IL-6, IL-10, and TNF-α relative to wild-type B cells. LPS stimulation increased IL-6 mRNA expression by B cells from wild-type and mutant mice in a dose-dependent manner. Similarly, although not in a dose-dependent manner, IL-10, TGF-β, and TNF-α mRNA levels also increased by LPS stimulation. Furthermore, levels of IL-6, IL-10, and TGF-β mRNA correlated with CD19 expression levels. Consistently, double staining of B cells in the skin tissues using anti-B220 (stained brown) and IL-10 (stained green) Abs revealed that IL-10 expression was decreased in CD19−/− mice and increased in CD19Tg mice relative to wild-type mice (Figure 4B). TNF-α mRNA levels were increased by CD19 overexpression but not significantly reduced by CD19 loss, regardless of LPS stimulation (Figure 4A). None of bFGF, aFGF, and PDGF was detected in B cells even after stimulation with LPS (data not shown). Thus, LPS-induced IL-6, IL-10, and TGF-β production by B cells generally correlated with CD19 expression levels.
Figure 4.
Cytokine production by wild-type (WT), CD19−/− (KO), and CD19Tg (TG) B cells stimulated with TLR4 ligands. A: Cytokine mRNA expression by B cells stimulated with LPS. B: B cells within wounds were double-stained with anti-B220 Ab (brown) and anti-IL-10 Ab (green; magnification = original ×1000). C: Cytokine mRNA expression by B cells stimulated with endogenous TLR4 ligands including hyaluronan (HA), fibrinogen (Fib), and heparan sulfate (HS). Relative mRNA expression of IL-6, IL-10, TGF-β, and TNF-α was quantified by real-time PCR and normalized relative to endogenous glyceraldehyde-3-phosphate dehydrogenase levels. D: Cytokine protein production by B cells stimulated with HA. E: IL-6 mRNA expression and protein production by wild-type B cells stimulated with dermatan sulfate (DS), chondroitin sulfate (CS), and HA. F: Inhibition of HA-induced cytokine mRNA expression and protein production by anti-TLR4 Ab. Wild-type B cells were pre-incubated with anti-TLR4 Ab (TLR4 Ab) as well as rat IgG2a control Ab (CTL Ab) before HA stimulation. IL-6, IL-10, and TGF-β mRNA expression was quantified by real-time PCR. Concentration of these cytokines was analyzed using specific ELISA. Each histogram shows the mean ± SEM values obtained from 6 mice in each group. *P < 0.05; **P < 0.01; #P < 0.05; ##P < 0.01 versus unstimulated B cells of the same genotype.
Cytokine Production by B Cells Stimulated with Endogenous Ligands for TLR
The recent identification of endogenous ligands of TLRs, including HA, heparan sulfate (HS), and fibrinogen, indicates that they function not only to induce defensive antimicrobial immune responses, but also as a sensitive detection system to initiate tissue regeneration after injury.23 Both HA and HS are glycosaminoglycan component of the extracellular matrix, and are degraded by proteases in inflammatory conditions.23,24,25 These fragmented HA and HS can signal through TLR4 and/or TLR2, which is capable of causing the release of pro-inflammatory cytokines from macrophages, dendritic cells, and endothelial cells.24,25,26,27,28,29,30 Similarly, fibrinogen, which leaks from the vasculature in response to endothelial cell retraction at sites of inflammation, activates TLR4.31 Thus, it is likely that B cells in wound site are exposed to HA, HS, and fibrinogen in a sterile condition. Therefore, we next examined whether B cells could produce cytokines by stimulation with endogenous TLR ligands, HA, HS, and fibrinogen in CD19-dependent manner like LPS (Figure 4C). In general, the pattern and intensity of cytokine mRNA expression by HA stimulation were similar to those by LPS stimulation, except for higher IL-10 mRNA expression by HA than by LPS (Figure 4C). The stimulatory effect by fibrinogen and HS was generally lower than that by HA except for the effect by HS for IL-6. Furthermore, unlike HA stimulation, neither fibrinogen nor HS stimulation augment TGF-β mRNA levels relative to the unstimulated status.
We next examined the protein levels of IL-6, IL-10, TGF-β, and TNF-α by HA-treated B cells (Figure 4D). Similar to the pattern of mRNA expression levels, the protein levels of IL-6, IL-10, and TGF-β by HA were also dependent on CD19 expression level. By contrast, other glycosaminoglycans, including dermatan sulfate and chondroitin sulfate did not affect any cytokine production by B cells examined in this study (Figure 4E and data not shown).
Then, whether IL-6, IL-10, and TGF-β production by B cells stimulated with HA was inhibited by anti-TLR4 Ab was assessed (Figure 4F). Wild-type B cells treated with HA and anti-TLR4 Ab exhibited reduced mRNA expression and protein production of IL-6, IL-10, and TGF-β relative to those treated with HA alone (54% to 74% decrease). By contrast, control Ab did not affect cytokine production. Thus, endogenous TLR ligands, especially HA, stimulated B cells to produce IL-6, IL-10, and TGF-β mainly through TLR4 in a CD19-depnedent manner.
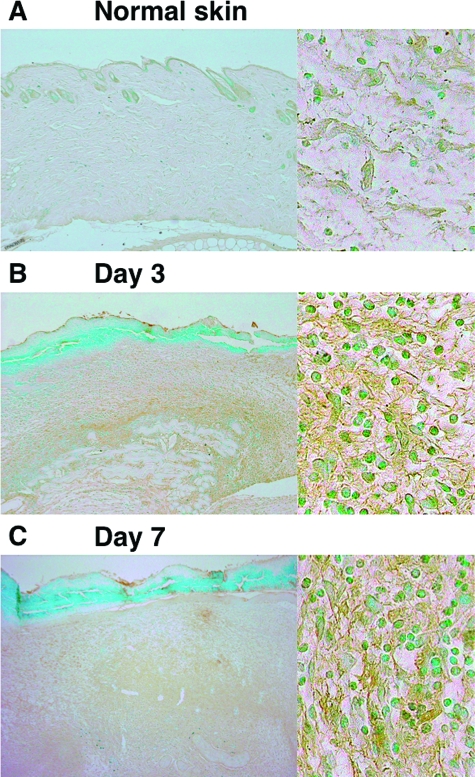
HA Accumulation in Wounded Skin
HA is one of the major structural components of the extracellular matrix, and undergoes rapid degradation at sites of tissue injury and inflammation.23 Therefore, HA composition was assessed immunohistochemically in normal and wounded skin (Figure 5). HA was slightly stained in normal skin (Figure 5A). HA staining greatly increased in wounded skin at both 3 and 7 days after injury compared with normal skin (Figure 5, B and C, left part). In addition, HA staining was mainly observed extracellularly (Figure 5, B and C, right part). There was no difference in HA accumulation between wild-type, CD19−/−, and CD19Tg mice (data not shown). Thus, HA was accumulated extracellularly in the injured skin tissue.
Figure 5.
HA accumulation in wild-type mice during wound healing. HA accumulation in normal skin (A) and in wounded skin at 3 days (B) and 7 days (C) after injury was assessed by immunohistochemistry using biotinylated HA binding protein (magnification = original ×40 and ×400). There was no difference in HA accumulation between wild-type, CD19−/−, and CD19Tg mice.
Expression of bFGF mRNA by Macrophages Stimulated with TGF-β
Expression of bFGF was reduced in the wounded skin from CD19−/− mice (Figure 3); however, wild-type B cells as well as CD19−/− B cells stimulated with LPS did not produce bFGF (data not shown). We hypothesized that skin decreased bFGF expression was related to reduced ability of macrophages to produce bFGF due to decreased cytokine production by CD19−/− B cells. Therefore, macrophages from wild-type mice were cultured with IL-6, IL-10, or TGF-β, and mRNA and protein levels of bFGF were examined by real-time PCR and ELISA (Figure 6, A and B). Both mRNA and protein levels of bFGF by macrophages were increased by TGF-β stimulation at both 1 and 10 ng/ml. By contrast, neither IL-6 nor IL-10 stimulation affects bFGF mRNA and protein levels. In addition, there was no significant difference in bFGF production levels by macrophages between wild-type and CD19 mutant mice (data not shown). Thus, TGF-β but not IL-6 and IL-10 increased bFGF production by macrophages.
Figure 6.
bFGF mRNA expression and protein production by macrophages stimulated with cytokines. Macrophages were purified from wild-type mice, and stimulated with either media alone, TGF-β, IL-6, or IL-10. A: Expression of bFGF mRNA were quantified by real-time PCR. B: Concentration of bFGF protein was analyzed by specific ELISA. Each histogram shows the mean ± SEM values obtained from six mice in each group.
Cell Proliferation Assay of Keratinocytes and Fibroblasts
bFGFs are mitogenic for several cell types present at the wound site, including fibroblasts and keratinocytes.32 We next assessed whether bFGF-induced cellular proliferation of keratinocytes and fibroblasts was affected by CD19 deficiency or overexpression (Table 1). After bFGF stimulation, keratinocyte proliferation was significantly increased in wild-type, CD19−/−, and CD19Tg mice at similar levels (2.16-, 2.19-, and 2.09-fold increase; respectively; P < 0.05). Similarly, fibroblast proliferation was significantly increased in wild-type, CD19−/−, and CD19Tg mice (2.66-, 2.63-, and 2.68-fold increase; respectively; P < 0.05). Thus, bFGF-induced cellular proliferation of keratinocytes and fibroblasts was not affected by CD19 expression.
Table 1.
In vitro Keratinocyte and Fibroblast Proliferative Responses in Wild-Type and CD19 Mutant Mice
| Proliferative responses (OD)
|
||||
|---|---|---|---|---|
| Cell types | Stimulation | Wild-type | CD19−/ − | CD19Tg |
| Keratinocytes | − | 0.32 ± 0.041 | 0.31 ± 0.055 | 0.33 ± 0.049 |
| bFGF | 0.69 ± 0.070* | 0.68 ± 0.074* | 0.69 ± 0.069* | |
| Fibroblasts | − | 0.41 ± 0.042 | 0.40 ± 0.048 | 0.41 ± 0.059 |
| bFGF | 1.09 ± 0.115* | 1.05 ± 0.090* | 1.10 ± 0.153* | |
Primary keratinocytes and fibroblasts isolated from wild-type and CD19 mutant mice were cultured for 72 hours with or without bFGF. BrdU was added during the last 24 hours of culture, and BrdU incorporation was assayed by ELISA. Values represent the mean OD (±SEM) obtained from six mice in each group.
P < 0.05 versus control of the same genotype.
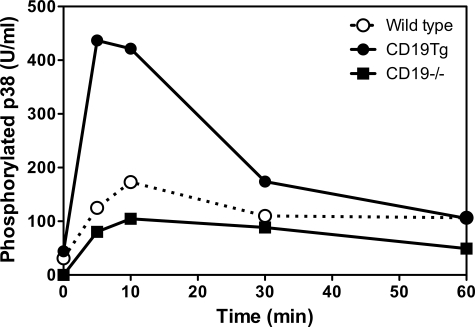
Regulation of LPS Signaling by CD19 through MAPK Activation
TLR signaling is closely linked to the MAPK signaling pathway that is also regulated by CD19 signaling.33,34 Therefore, we examined whether CD19 expression altered MAPK signaling transduction mediated by LPS stimulation (Figure 7). CD19 expression did not affect c-Jun N-terminal kinase and extracellular signal-regulated kinase (data not shown). B cells from CD19Tg mice showed enhanced phosphorylated p38 level by LPS stimulation relative to those from wild-type mice. By contrast, B cells from CD19−/− mice showed modest p38 phosphorylation level by LPS stimulation. Thus, CD19 expression regulated p38-signaling transduction mediated by LPS stimulation.
Figure 7.
p38 MAPK phosphorylation induced by LPS stimulation. Splenic B cells from wild-type, CD19−/−, and CD19Tg were incubated with LPS (10 μg/ml) for the indicated time, and subsequently lysed. p38 phosphorylation levels were determined by ELISA. These results are representative of those obtained in three independent experiments.
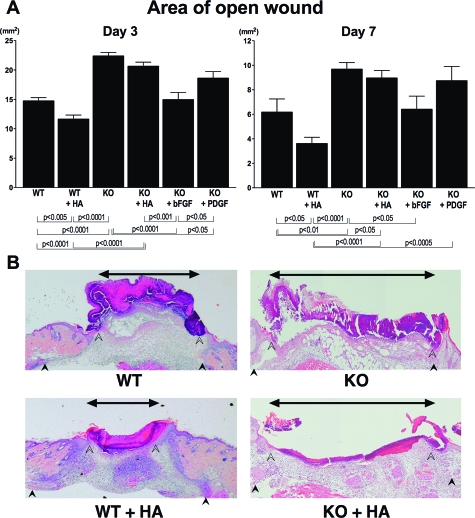
Effect of Topical Administration of Growth Factors and HA on Wound Healing
Finally, we examined the effect of HA on delayed wound healing observed in CD19−/− mice, and compared this effect with that of growth factors (Figure 8, A and B). At 3 and 7 days after injury, administration of HA promoted wound repair in wild-type mice. By contrast, open wound area was not changed in CD19−/− mice by the addition of HA (Figure 8B). Administration of bFGF normalized the delayed wound healing by 3 days after injury. However, PDGF application did not normalize the delayed wound repair after 3 and 7 days. Thus, topical administration of HA promoted wound repair in wild-type mice but not in CD19−/− mice.
Figure 8.
Effect of growth factors or HA on delayed wound repair in CD19−/− mice. Growth factors (bFGF, 1000 ng; PDGF, 800 ng) or HA (20 μg) were applied to each wound in 20-μl aqueous buffer immediately and 12 hours after wounding, and wounds were covered with an occlusive dressing (Tegaderm, 3M Canada). A: Macroscopic area of the open wound was measured at 3 and 7 days after wounding. Each histogram shows the mean ± SEM values obtained from 15 mice (50 wounds) in wild-type group, 14 mice (42 wounds) in CD19−/− and CD19Tg group, and 10 mice (38 wounds) in groups of CD19−/− treated with HA or growth factors. B: Representative histology of wound tissue at 3 days after wounding in wild-type (WT) mice and CD19−/− (KO) mice treated with HA. Edges of the epithelium and panniculus carnosus were indicated by open and filled arrowheads. Double pointed arrows denote epithelial gap.
Discussion
The current study is the first to demonstrate that B cells play a critical role in the wound-healing process. Macroscopic wound healing, re-epithelialization, and granulation tissue formation were inhibited due to CD19 loss by 3 days after wounding (Figure 1). By contrast, CD19Tg mice exhibited enhanced re-epithelialization and granulation tissue formation by 3 days after wounding (Figure 1). These results indicate that CD19 expression positively regulates the wound-healing process. Delayed wound healing in CD19−/− mice was associated with decreased infiltration of neutrophils and macrophages (Figure 2, A, B, and D). In addition, CD19 expression also regulated skin expression of cytokines, such as bFGF, aFGF, IL-6, IL-10, PDGF, and TGF-β, especially at 3 days after injury (Figure 3). Furthermore, HA as well as LPS stimulated B cells, which infiltrated into wounds (Figure 2, C and E), to produce several cytokines through TLR4 in a CD19-dependent manner (Figure 4). Wounding induced significant local HA accumulation (Figure 5). Taken together, the results of the present study indicate that CD19 expression controls cytokine production by B cells through TLR4 activation and thereby regulates wound-healing process.
The loss or overexpression of CD19 affected wound-healing process by 3 days after injury (Figure 1), suggesting that B cells play a critical role in early stage of the wound-healing process. CD19 regulates both BCR and TLR signaling.14,15 However, adaptive immune responses, through BCR requires gene rearrangement and its response, is inevitably delayed. By contrast, the innate immune system through TLRs can respond microbial pathogens promptly and provides the first line of host defense. Therefore, we hypothesized that B cells regulate the wound-healing process by producing various cytokines through activation of innate immune system and that CD19 controls these cytokine levels. Consistent with this possibility, B cells produced various cytokines, including IL-6, IL-10, and TGF-β, by LPS stimulation in a CD19-dependent manner (Figure 4A). However, as normal wound-repair process occurs in the absence of microbial stimuli such as LPS, we examined the role of several endogenous ligands for TLRs including HA, fibrinogen, and HS as a trigger of sterile injury. Remarkably, among them, HA most strongly stimulated B cells to produce cytokines with similar pattern and intensity of LPS, which was also dependent on CD19 expression (Figure 4C). Previous studies have suggested that trauma can release small molecular weight HA fragments from the extracellular matrix, thus potentially presenting HA as an intrinsic signal of inflammation.25 Indeed, increased HA accumulation was observed in injured skin tissue (Figure 5). Collectively, the influence of HA on B cell functions would provide novel mechanisms how CD19 expression affects wound healing process: under the sterile injury, increased HA accumulation in the wounds may induce cytokine production by B cells CD19-dependently and thereby regulates wound healing process.
It has been reported that the application of exogenous HA enhances keratinocyte proliferation both in vitro and in vivo.35 Consistent with this, treatment with HA enhanced wound healing in wild-type mice by 3 days after wounding. By contrast, HA application tended to improve delayed wound healing observed in CD19−/− mice, but could not normalize it (Figure 8). Many cells at the wound site express CD44, which is known as a HA receptor, and it is possible that HA-CD44 signaling may affect skin wound healing. However, it has become apparent from previous studies in CD44-null mice that although CD44 is essential for regulating HA turnover, it is not required for expression of chemokines or cytokines by macrophages in vivo.36,37 By contrast, the expression of these chemokines is decreased in TLR4−/− or MyD88−/− mice.28,38 Thus, it is likely that TLR4-signaling is more important for HA-mediated cytokine or growth factor expression in the wound healing process than CD44. In addition, a previous study has revealed that cytokine profiles due to TLR4 activation are different between B cells and macrophages.39 Therefore, it is possible that although the number of B cells within a wound is small, the inadequate increase of B cell-derived cytokines in CD19−/− mice fails to create the proper cytokine network, resulting in little effect on the healing response by HA application. Collectively, these results suggest that although immune cells other than B cells, such as macrophages, have TLR4 and produce inflammatory cytokines in response to HA,25,26,27,28 proper B cell response is indispensable for improving wound healing.
Treatment with anti-TLR4 Ab did not completely suppress cytokine production by B cells stimulated with HA (Figure 4F). A recent study has shown that HA stimulates macrophage chemokine production in a TLR4- and TLR2-dependent manner.28 Murine B cells express both TLR2 and TLR4.40 In addition, CD44 can also serve as a HA receptor resulting in B cell activation.41 Therefore, the residual cytokine production after TLR4 blockade may be due to either TLR2 or CD44 activation by HA. Furthermore, cytokine production by HA-stimulated B cells was regulated by CD19 expression, suggesting that CD19 influences TLR4 signaling (Figure 4C). Indeed, a previous study has shown that CD19−/− B cells exhibit reduced proliferation in response to LPS stimulation, while CD19Tg mice show augmented proliferation.42 Furthermore, tyrosine phosphorylation of p38, which is downstream of TLR4 signaling pathway,34 was controlled by CD19 expression (Figure 7). Thus, CD19 regulates TLR4 signaling in B cells and thereby controls TLR4-induced cytokine production that promotes wound-healing process.
There are two possibilities for how CD19 affects the B cell response by HA. It was reported that CD19 regulated signaling pathway of RP105, another member of TLR and homolog molecule of TLR4.43 Therefore, HA might also stimulate RP105 signaling, and thereby HA stimulation was controlled by CD19. Alternatively, TLR4 signaling may be indirectly regulated by CD19. Recently, it has been reported that RP105−/− B cells proliferate poorly in response to not only the TLR4 ligand LPS but also TLR2 ligand lipoproteins, and that RP105/MD-1 controls Ab production mediated via TLR2 and TLR4/MD-2 receptor complex. Therefore, the loss of CD19 may indirectly decrease TLR2 and/or TLR4 signaling by inhibiting RP105 signaling, which resulted in reduced response to HA stimulation. However, we did not assess whether HA can bind to RP105 or whether CD19-dependent B cell cytokine expression was abolished by blocking RP105. Therefore, further research will be required to address these issues.
In the current study, bFGF, aFGF, IL-6, IL-10, PDGF, and TGF-β mRNA levels were decreased in the wounded skin from CD19−/− mice (Figure 3). Both aFGF and bFGF enhance wound healing: FGFs are mitogenic for several cell types present at the wound site, including fibroblasts and keratinocytes.32 Indeed, bFGF−/− mice exhibit delayed wound healing to full-thickness excisional wound.44 IL-6 also has critical roles in wound healing, since IL-6−/− mice show impaired skin wound healing probably by regulating leukocyte infiltration, angiogenesis, and collagen accumulation.45,46 Furthermore, TGF-β promotes re-epithelialization of skin wounds of rats47 and stimulates epidermal outgrowth in vitro.48 PDGF also augments deposition of provisional wound matrix, collagen synthesis by fibroblasts, and the acute inflammatory response.49,50 Therefore, the decreased expression of bFGF, aFGF, IL-6, TGF-β, and PDGF may cooperatively delayed wound healing in CD19−/− mice (Figure 3). By contrast, IL-10 likely impedes wound repair via decreasing macrophage infiltration.51 Thus, CD19 deficiency inhibited not only production of cytokines that stimulates wound healing, but also production of IL-10 that inhibits wound repair, suggesting that delayed wound healing by CD19 deficiency reflects a net effect of complicated cytokine functions.
In the current study, topical bFGF treatment completely normalized delayed wound healing in CD19−/− mice; however, PDGF was not sufficient to normalize it (Figure 8). Therefore, decreased skin bFGF expression may be predominantly related to the delayed wound healing in CD19−/− mice. However, change in bFGF alone did not explain the accelerated wound repair in CD19Tg mice, since bFGF expression was not significantly increased (Figure 3). Although B cells did not produce bFGF (data not shown), macrophages produced bFGF by TGF-β stimulation (Figure 6), suggesting that decreased bFGF expression in skin is related to reduced ability of macrophages of producing bFGF due to decreased TGF-β production by CD19−/− B cells. This finding also suggests complex interaction between immune cells and cytokines. Although CD19Tg mice showed enhanced mRNA levels of aFGF, PDGF, and TGF-β, IL-6 mRNA levels were not significantly increased after 3 and 7 days (Figure 3). This may account for the failure of enhanced macroscopic wound healing in CD19Tg mice (Figure 1). Collectively, these results suggest that altered expression of bFGF, aFGF, IL-6, IL-10, PDGF, and TGF-β by the loss or overexpression of CD19 affects the wound healing process directly or in part through interaction with other immune cells and skin residual cells.
Despite the significant difference in inflammatory cell infiltration and cytokine expression within wounds, there was no difference in B cell numbers (Figures 2 and 3). There are two possible reasons for this. One possibility is decreased peripheral B cell numbers in CD19Tg mice. It is known that CD19Tg B cells are hyperresponsive to transmembrane signals and proliferate at elevated levels; however, there is a >95% reduction in the number of B cells exiting the bone marrow and entering the circulating B cell pool, presumably the result of enhanced negative selection during development.14,16,52 Another possibility is increased spontaneous apoptosis in CD19Tg B cells. When B cells are activated, CD95 is up-regulated and its interaction with CD95 ligand on T cells induces CD95-mediated apoptosis (activation-induced cell death), which is essential in the down-regulation of B cell activation.53 Indeed, in B cells from systemic sclerosis, which also overexpress CD19 and are hyperresponsive to various stimulation, CD95 is up-regulated and spontaneous apoptosis in peripheral B cells are increased.54 Therefore, although CD19Tg B cells produced increased levels of cytokines and induced increased inflammation, spontaneous apoptosis may also be increased. Inversely, CD19−/− B cells produced decreased levels of cytokines levels, and spontaneous apoptosis may also be decreased relative to wild-type mice.
In summary, we propose a novel mechanism how B cells regulate wound healing: HA, which was produced during wound healing process, stimulates B cells through TLR4 to produce various cytokines and growth factors that cooperatively promote wound healing in part by activating other immune cells. CD19 is suggested to regulate the contribution of B cells to wound healing by affecting TLR4 signaling and thereby altering cytokine production. Understanding regulation of cutaneous wound repair is important as there are many disorders based on abnormal wound repair, including stasis ulcer, diabetic ulcer, keloids, and hypertrophic scars.5 Understanding the contributions of B cells to the wound repair process could provide new clues to regulating wound healing.
Acknowledgments
We thank Ms. Yuko Yamada, Masako Matsubara, Aya Usui, Mariko Yozaki, and Kaori Shimoda for technical assistance.
Footnotes
Address reprint requests to Dr. Shinichi Sato, Department of Dermatology, Nagasaki University Graduate School of Biomedical Sciences, 1-7-1 Sakamoto, Nagasaki, 852-8501, Japan. E-mail: s-sato@nagasaki-u.ac.jp.
Supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and The Nakatomi Foundation (to S.S.) and National Institutes of Health grants CA96547, CA105001, and AI56363 (to T.F.T.).
References
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–556. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–528. doi: 10.1016/s0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- Blotnick S, Peoples GE, Freeman MR, Eberlein TJ, Klagsbrun M. T lymphocytes synthesize and export heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblasts: differential production and release by CD4+ and CD8+ T cells. Proc Natl Acad Sci USA. 1994;91:2890–2894. doi: 10.1073/pnas.91.8.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer M, Barbul A. Lymphocyte function in wound healing and following injury. Br J Surg. 1998;85:444–460. doi: 10.1046/j.1365-2168.1998.00734.x. [DOI] [PubMed] [Google Scholar]
- Boyce DE, Jones WD, Ruge F, Harding KG, Moore K. The role of lymphocytes in human dermal wound healing. Br J Dermatol. 2000;143:59–65. doi: 10.1046/j.1365-2133.2000.03591.x. [DOI] [PubMed] [Google Scholar]
- Cowin AJ, Brosnan MP, Holmes TM, Ferguson MW. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev Dyn. 1998;212:385–393. doi: 10.1002/(SICI)1097-0177(199807)212:3<385::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Richards AM, Floyd DC, Terenghi G, McGrouther DA. Cellular changes in denervated tissue during wound healing in a rat model. Br J Dermatol. 1999;140:1093–1099. doi: 10.1046/j.1365-2133.1999.02908.x. [DOI] [PubMed] [Google Scholar]
- Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2:764–766. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- Sato S, Hasegawa M, Fujimoto M, Tedder TF, Takehara K. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J Immunol. 2000;165:6635–6643. doi: 10.4049/jimmunol.165.11.6635. [DOI] [PubMed] [Google Scholar]
- Engel P, Zhou LJ, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- Sato S, Steeber DA, Jansen PJ, Tedder TF. CD19 expression levels regulate B lymphocyte development: human CD19 restores normal function in mice lacking endogenous CD19. J Immunol. 1997;158:4662–4669. [PubMed] [Google Scholar]
- Zhou LJ, Smith HM, Waldschmidt TJ, Schwarting R, Daley J, Tedder TF. Tissue-specific expression of the human CD19 gene in transgenic mice inhibits antigen-independent B-lymphocyte development. Mol Cell Biol. 1994;14:3884–3894. doi: 10.1128/mcb.14.6.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M, Saffaripour S, Van De Water L, Frenette PS, Mayadas TN, Hynes RO, Wagner DD. Role of endothelial selectins in wound repair. Am J Pathol. 1997;150:1701–1709. [PMC free article] [PubMed] [Google Scholar]
- Meijerink J, Mandigers C, van de Locht L, Tonnissen E, Goodsaid F, Raemaekers J. A novel method to compensate for different amplification efficiencies between patient DNA samples in quantitative real-time PCR. J Mol Diagn. 2001;3:55–61. doi: 10.1016/S1525-1578(10)60652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwali A, Blum AM, Elliott DE, Weinstock JV. IL-4 inhibits vasoactive intestinal peptide production by macrophages. Am J Physiol Gastrointest Liver Physiol. 2002;283:G115–G121. doi: 10.1152/ajpgi.00491.2001. [DOI] [PubMed] [Google Scholar]
- Arsenescu R, Blum AM, Metwali A, Elliott DE, Weinstock JV. IL-12 induction of mRNA encoding substance P in murine macrophages from the spleen and sites of inflammation. J Immunol. 2005;174:3906–3911. doi: 10.4049/jimmunol.174.7.3906. [DOI] [PubMed] [Google Scholar]
- Marcelo CL, Kim YG, Kaine JL, Voorhees JJ. Stratification, specialization, and proliferation of primary keratinocyte cultures. Evidence of a functioning in vitro epidermal cell system. J Cell Biol. 1978;79:356–370. doi: 10.1083/jcb.79.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schluesener HJ. Mammalian toll-like receptors: from endogenous ligands to tissue regeneration. Cell Mol Life Sci. 2006;63:2901–2907. doi: 10.1007/s00018-006-6189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GB, Brunn GJ, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J Immunol. 2004;172:20–24. doi: 10.4049/jimmunol.172.1.20. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Kodaira Y, Nair SK, Wrenshall LE, Gilboa E, Platt JL. Phenotypic and functional maturation of dendritic cells mediated by heparan sulfate. J Immunol. 2000;165:1599–1604. doi: 10.4049/jimmunol.165.3.1599. [DOI] [PubMed] [Google Scholar]
- Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Li X, Carter RH. Convergence of CD19 and B cell antigen receptor signals at MEK1 in the ERK2 activation cascade. J Immunol. 1998;161:5901–5908. [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Price RD, Berry MG, Navsaria HA. Hyaluronic acid: the scientific and clinical evidence. J Plast Reconstr Aesthet Surg. 2007;60:1110–1119. doi: 10.1016/j.bjps.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- Macedo L, Pinhal-Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol. 2007;171:1774–1788. doi: 10.2353/ajpath.2007.061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Sfady M, Nussbaum G, Pevsner-Fischer M, Mor F, Carmi P, Zanin-Zhorov A, Lider O, Cohen IR. Heat shock protein 60 activates B cells via the TLR4-MyD88 pathway. J Immunol. 2005;175:3594–3602. doi: 10.4049/jimmunol.175.6.3594. [DOI] [PubMed] [Google Scholar]
- Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafi A, Nagarkatti M, Nagarkatti PS. Hyaluronate-CD44 interactions can induce murine B-cell activation. Blood. 1997;89:2901–2908. [PubMed] [Google Scholar]
- Sato S, Ono N, Steeber DA, Pisetsky DS, Tedder TF. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J Immunol. 1996;157:4371–4378. [PubMed] [Google Scholar]
- Yazawa N, Fujimoto M, Sato S, Miyake K, Asano N, Nagai Y, Takeuchi O, Takeda K, Okochi H, Akira S, Tedder TF, Tamaki K. CD19 regulates innate immunity by the toll-like receptor RP105 signaling in B lymphocytes. Blood. 2003;102:1374–1380. doi: 10.1182/blood-2002-11-3573. [DOI] [PubMed] [Google Scholar]
- Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci USA. 1998;95:5672–5677. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, Luster MI. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14:2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73:713–721. doi: 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- Mustoe TA, Pierce GF, Thomason A, Gramates P, Sporn MB, Deuel TF. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987;237:1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Hebda PA. Stimulatory effects of transforming growth factor-beta and epidermal growth factor on epidermal cell outgrowth from porcine skin explant cultures. J Invest Dermatol. 1988;91:440–445. doi: 10.1111/1523-1747.ep12476480. [DOI] [PubMed] [Google Scholar]
- Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, Deuel TF. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989;109:429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GF, Tarpley JE, Yanagihara D, Mustoe TA, Fox GM, Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-beta 1, and basic fibroblast growth factor in dermal wound healing. Neovessel and matrix formation and cessation of repair. Am J Pathol. 1992;140:1375–1388. [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Werner S, Bugnon P, Wickenhauser C, Siewe L, Utermohlen O, Davidson JM, Krieg T, Roers A. Accelerated wound closure in mice deficient for interleukin-10. Am J Pathol. 2007;170:188–202. doi: 10.2353/ajpath.2007.060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Poe JC, Hasegawa M, Tedder TF. CD19 regulates intrinsic B lymphocyte signal transduction and activation through a novel mechanism of processive amplification. Immunol Res. 2000;22:281–298. doi: 10.1385/IR:22:2-3:281. [DOI] [PubMed] [Google Scholar]
- Wang J, Watanabe T. Expression and function of Fas during differentiation and activation of B cells. Int Rev Immunol. 1999;18:367–379. doi: 10.3109/08830189909088489. [DOI] [PubMed] [Google Scholar]
- Sato S, Fujimoto M, Hasegawa M, Takehara K. Altered blood B lymphocyte homeostasis in systemic sclerosis: expanded naive B cells and diminished but activated memory B cells. Arthritis Rheum. 2004;50:1918–1927. doi: 10.1002/art.20274. [DOI] [PubMed] [Google Scholar]