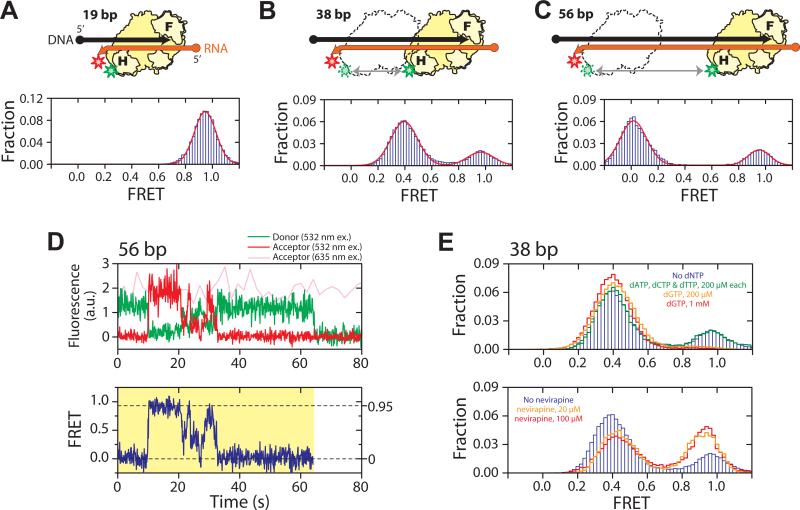
Fig. 1.
RT slides on nucleic acid substrates. (A - C) H-labeled RT (yellow) bound to a 19 bp (A), 38 bp (B), and 56 bp (C) back-labeled DNA (black) / RNA (orange) hybrid. F and H on the enzyme represent the fingers and RNase H domains, and circle and arrow on the nucleic acid strands represent 5′ and 3′ ends, respectively. Green and red stars indicate Cy3 and Cy5 dyes, respectively. The FRET histograms (blue bars) were fit with a single (A) or double (B, C) Gaussian peaks (red line). (D) Representative fluorescence (upper panel) and FRET (lower panel) time traces of RT bound to a 56 bp DNA/RNA hybrid at 12 °C, showing gradual transitions between the 0 and 0.95 FRET states and preferred intermediates near FRET ∼ 0.3 − 0.5. The time resolution is 10 Hz. The green and red traces represent donor and acceptor fluorescence under 532 nm excitation and the pink trace represents acceptor fluorescence under 635 nm excitation. The yellow shade marks a single RT binding event. (E) Effects of nucleotides and nevirapine on sliding. (Upper panel) FRET histograms of RT bound to the 38 bp DNA/RNA hybrid in the absence of nucleotides (blue bars), in the presence of the cognate dGTP (orange and red lines), or non-cognate nucleotides (green line). The DNA primer was chain-terminated to prevent elongation. (Lower panel) FRET histograms in the absence (blue bars) and presence of nevirapine (orange and red lines).