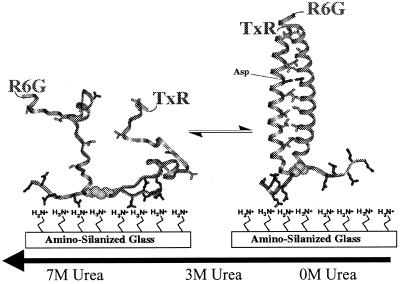
Figure 1.
Schematic representation of the folding of GCN4-Pf. (Right) Structure of folded GCN4-P1 from x-ray diffraction.(13). A hypothetical unfolded structure is shown at Left. The peptide adheres to the positively charged surface by electrostatic interaction with the negatively charged glutamic acids at the C terminus of the peptide. Conformational fluctuations cause changes in the donor–acceptor distance, resulting in an anticorrelated modulation in the donor and acceptor fluorescence intensities.